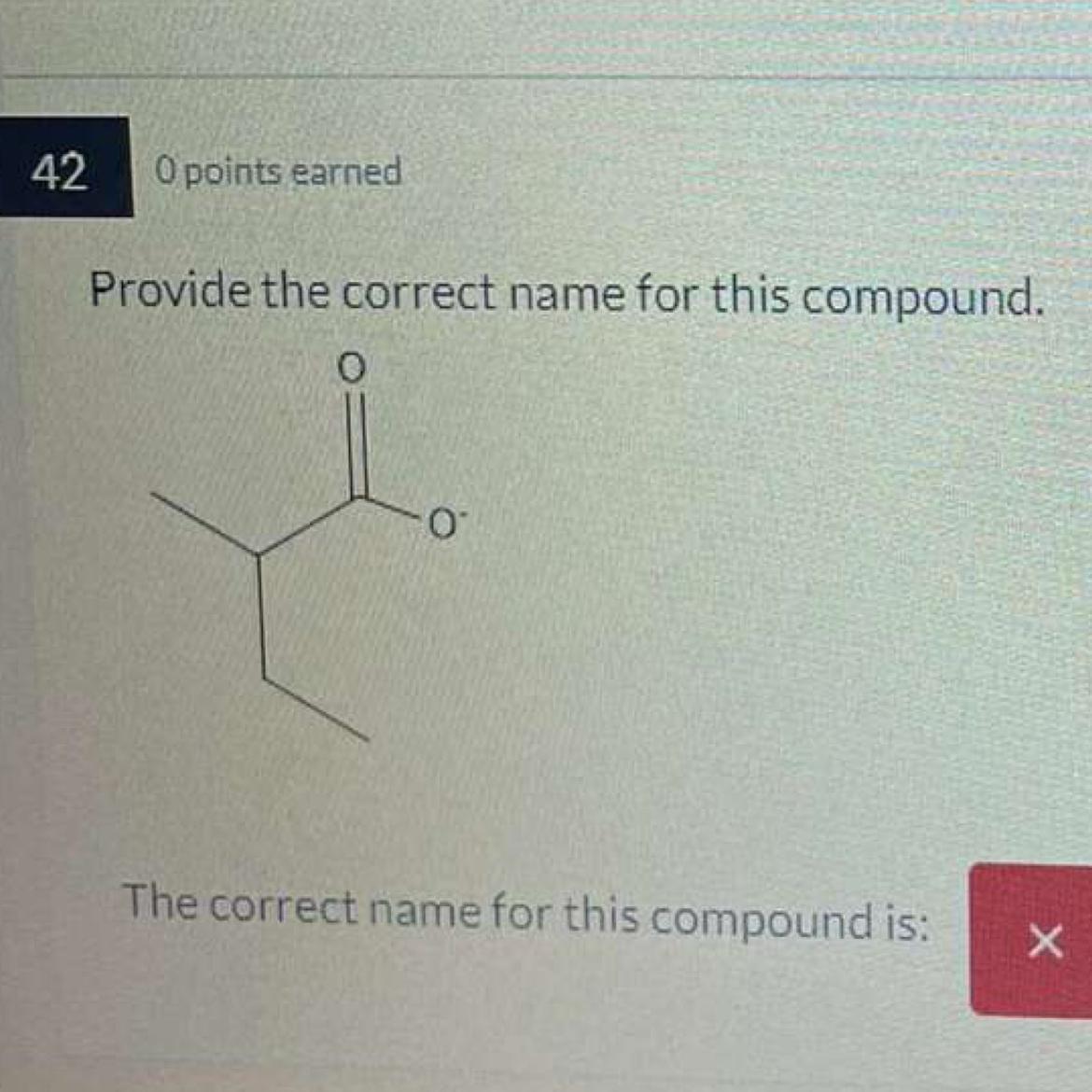
Answers
Methyl 2-ethylbutyrate is the proper name for this substance in the given figure.
A formal condensation between ethanol and 2-methylbutyric acid produces ethyl 2-methylbutyrate, a fatty acid ethyl ester. Wines, strawberries, blueberries, and apples all include it as a component of their scent. It serves as a flavoring ingredient as well as a plant metabolite, human metabolite, and fragrance.
Is butyl ethyl ester an ester?With the chemical formula CH3CH2CH2COOCH2CH3, ethyl butyrate, commonly referred to as ethyl butanoate or butyric ether, is an ester. It is soluble in kerosene, paraffin oil, and propylene glycol. It is a significant element used to enhance the flavor of processed orange juices and has a delicious aroma reminiscent of pineapple.
To know more about ester visit:-
https://brainly.com/question/10840252
#SPJ13
Related Questions
Why should precision volumetric glassware be rinsed with distilled water after use?
Answers
Answer:
it should be rinsed to clean the glassware
Photoelectric effect will occur only if frequency of light striking an electron in a metal is above a certain threshold frequenci
Answers
The statement is correct. The photoelectric effect refers to the phenomenon where electrons are ejected from the surface of a material when it is exposed to light. The frequency of light striking an electron in a metal must be above the threshold frequency in order for the photoelectric effect to occur.
The statement is correct. The photoelectric effect refers to the phenomenon where electrons are ejected from the surface of a material when it is exposed to light. However, for the photoelectric effect to occur, the frequency of the incident light must be above a certain threshold frequency.
The threshold frequency is the minimum frequency of light required to dislodge electrons from the material. Below this threshold frequency, regardless of the intensity or duration of the light, no electrons will be emitted.
This behavior can be explained by the particle-like nature of light, where light is composed of discrete packets of energy called photons. The energy of a photon is directly proportional to its frequency. Only photons with energy greater than or equal to the binding energy of the electrons in the material can dislodge them.
Therefore, the frequency of light striking an electron in a metal must be above the threshold frequency in order for the photoelectric effect to occur.
For more question on photoelectric
https://brainly.com/question/1458544
#SPJ8
4- The standard potential of cell: Sn/Sn²+||Cr³+/Cr is −0.60V.what is the standard
reduction potential of the Cr³+/Crelectrode? Es = -0.14V
Sn²+
(b) +0.74V
(c) -0.88V
(d) -0.74V
(a) +0.88V
Answers
The standard reduction potential of the Cr³+/Cr electrode is -0.46V. None of the option is correct.
To determine the standard reduction potential of the Cr³+/Cr electrode, we can use the Nernst equation, which relates the standard reduction potential to the cell potential under non-standard conditions. The Nernst equation is given by:
E = E° - (0.0592/n) * log(Q)
where E is the cell potential, E° is the standard reduction potential, n is the number of electrons transferred in the half-reaction, and Q is the reaction quotient.
In this case, we have the standard potential of the cell as −0.60V. We know that the standard reduction potential of the Sn/Sn²+ electrode is -0.14V. Therefore, the reduction potential of the Cr³+/Cr electrode can be calculated as:
E = -0.60V - (-0.14V)
E = -0.60V + 0.14V
E = -0.46V
Therefore, the standard reduction potential of the Cr³+/Cr electrode is -0.46V.
For more such question on standard reduction potential visit;
https://brainly.com/question/31482299
#SPJ8
please help
Determine the mass of hydrogen gas collected in a container if the gas occupies
44.8 L at STP.
Answers
The chemical formula for hydrogen sulfide is therefore H2S. The hydrogen sulfide mass would thus be 1/2+32=34g/mol at STP.
What exactly will be in a molecular formula?A chemical formula typically identifies the variety of atoms that make up a molecule is its molecular formula. For instance, the presence of two oxygen atoms in CO2 is indicated by the subscript beneath the oxygen atom, whereas the absence of a subscript under the carbon atom indicates that just one carbon atom is present.
What is a molecular formula, and give an example.The number of atoms from each element in a single chemical molecule is specified by the molecular formula, which is an equation. In a molecule, it displays the precise number of each atom. An illustration is propane, which has the chemical formula C4H10. The provided compound has a formula that reads as follows: 4 carbon atoms, 10 hydrogen atoms.
To know more about molecular formula visit:
https://brainly.com/question/28647690
#SPJ1
Use the data in the table below to calculate the heat of vaporization (AHvap) in kJ/mol of pinene.
Vapor Pressure
(torr)
760
515
340
218
135
Temperature
(K)
429
415
401
387
373
kJ/mol
Use the value of AHyap determined in Part 1 to calculate the vapor pressure of pinene (in torr) at room temperature (23°C)
760
torr

Answers
Answer:
41 kJ/mol4 torrExplanation:
Given pinene has a (temperature, vapor pressure) relation (K, torr) = {(373, 135), (429, 760)}, you want the heat of vaporization in kJ/mol and the vapor pressure at room temperature (23 °C).
Clausius–Clapeyron EquationThe Clausius–Clapeyron equation can be used to find the heat of vaporization:
\(\ln{P}=-\dfrac{\Delta H_{\text{vap}}}{R}\left(\dfrac{1}{T}\right)+C\)
Solving for ∆H, we find ...
\(\Delta H_{\text{vap}}=-\dfrac{R\cdot\ln{\dfrac{P_1}{P_2}}}{\dfrac{1}{T_1}-\dfrac{1}{T_2}}\\\\\\\Delta H_{\text{vap}}=-\dfrac{8.314\cdot\ln{\dfrac{760}{135}}}{\dfrac{1}{429}-\dfrac{1}{373}}\approx 41052.8\)
The heat of vaporization of pinene is about 41 kJ/mol.
Vapor pressureRearranging the above equation to give P1, we have ...
\(\ln{\dfrac{P_1}{P_2}}=-\dfrac{\Delta H_\text{vap}}{R}\left(\dfrac{1}{T_1}-\dfrac{1}{T_2}\right) \\\\\\P_1=P_2\cdot e^{-\frac{\Delta H_\text{vap}}{R}\left(T_1^{-1}-T_2^{-1})}\)
Using the same P2 and T2 as above, we find the vapor pressure at room temperature (296.15 K) to be ...
P1 ≈ 4.349 . . . . . torr
The vapor pressure of pinene at room temperature is about 4 torr.

determine moles of 1.5g of sodium carbonate.
Answers
Answer:
0.014mol
Explanation:
Given parameters:
Mass of Na₂CO₃ = 1.5g
Unknown:
Number of moles = ?
Solution:
Number of moles of a compound is mathematically expressed as;
Number of moles = \(\frac{mass}{molar mass}\)
Molar mass of Na₂CO₃ = 2(23) + 12 + 3(16) = 106g/mol
Number of moles = \(\frac{1.5}{106}\) = 0.014mol
what are thetypes of luminous flame
Answers
Types of luminous flames:
1. Yellow Luminous Flame
2. Smoky Luminous Flame
3. Orange Luminous Flame
4. Blue Luminous Flame
Luminous flames are characterized by their visible glow, which is caused by the incomplete combustion of fuel. The presence of soot particles in the flame causes the emission of light. There are different types of luminous flames, which can be classified based on their fuel composition and burning conditions. Here are some common types of luminous flames:
1. Yellow Luminous Flame: This is the most common type of luminous flame, often seen in open fires, candles, and gas stoves. It appears yellow due to the presence of soot particles in the flame. Yellow flames indicate incomplete combustion of hydrocarbon fuels, such as methane, propane, or natural gas. The high carbon content in these fuels leads to the formation of soot, which emits visible light.
2. Smoky Luminous Flame: This type of flame is characterized by a significant amount of black smoke and soot production. It is commonly observed in poorly adjusted or malfunctioning burners or engines. The excessive presence of unburned fuel in the flame results in incomplete combustion and the emission of dark smoke particles.
3. Orange Luminous Flame: An orange flame indicates a higher combustion temperature compared to a yellow flame. It is often seen in more efficient burners or when burning fuels with a higher carbon content, such as oil or diesel. The higher temperature helps in burning more of the carbon particles, reducing the amount of soot and making the flame appear less yellow.
4. Blue Luminous Flame: A blue flame is typically associated with complete combustion. It indicates efficient burning of fuel, resulting in minimal soot formation. Blue flames are commonly observed in gas burners or Bunsen burners. The blue color is a result of the combustion of gases, such as methane, in the presence of sufficient oxygen.
It's important to note that the luminosity of a flame can vary depending on factors such as fuel-air mixture, combustion temperature, and the presence of impurities. Achieving complete combustion and minimizing the production of soot is desirable for efficient and cleaner burning processes.
for more questions on luminous
https://brainly.com/question/27163038
#SPJ8
For the balanced reaction below, determine the limiting reactant if 1.5 moles of methane reactswith 2.0 moles of oxygen. Show all your workCH4(8) + 2O2(g) → CO2(g) + 2H2O(g)(methane)

Answers
ANSWER
Oxygen is the limiting reactant in the reaction
EXPLANATION
Given information
\(\text{ CH}_{4(g)}+\text{ 2O}_{2(g)}\rightarrow\text{ CO}_{2(g)}\text{ + 2H}_2O(g)\)The mole of methane is 1.5 moles
The mole of oxygen is 2.0 moles
To determine the limiting reactant in the reaction above, follow the steps below
Step 1: Write the formula for calculating the limiting reactant in a reaction
\(\text{ = }\frac{moles}{stoichiometric\text{ coefficient of the reactant}}\)Step 2: write the stoichiometric coefficient of the reactants in the above equation
The stoichiometric coefficient of methane is 1
The stoichiometric coefficient of oxygen is 2
Step 3: Divide the mole of the reactants by their stoichiometric coefficient
\(\begin{gathered} \text{ Methane = }\frac{1.5}{1} \\ \text{ Methane = 1.5 mol/wt } \\ \\ \text{ Oxygen = }\frac{2.0}{2} \\ \text{ oxygen = 1 mol/wt} \end{gathered}\)From the above calculations, you will see that oxygen has the least mole per weight.
Therefore, oxygen is the limiting reactant in the above reaction
Hey can someone help me fill out these blanks

Answers
Answer:
Constructive interference is when two waves interact causing larger waves because the crest will meet a crest or a trough will meet a trough which adds the waves together changing the size of the amplitude.
Destructive interference is when two waves interact causing smaller waves because the crest will meet a trough or a trough will meet a crest which subtracts the waves together changing the size of the amplitude.
The blue color in fireworks is often achieved by heating CuCl to about 1200°C. The compound emits blue light having a wavelength of 450nm. What is the increment of energy (the guatum) that is emitted at 4.50 *10²nm by CuCl
Answers
The increment of energy (or quantum) that is emitted at 450nm by CuCl is 5.93 x 10⁻¹⁶ J.
What is quantum?Quantum is a branch of physics that studies the behavior of matter and energy at the atomic and subatomic level. It uses quantum mechanics to describe and predict how particles behave and interact. This branch of physics is used to explain phenomena such as the nature of light, the structure of atoms, and the behavior of materials at extremely small scales.
The energy (in Joules) that is emitted at 450nm by CuCl is calculated using the equation E = hc/λ,
where h is Planck's constant (6.626 x 10⁻³⁴ J·s),
c is the speed of light (2.998 x 10⁸ m/s), and
λ is the wavelength of light in meters.
Plugging in these values gives us:
E = (6.626 x 10⁻³⁴ J·s) (2.998 x 10⁸ m/s) / (450 x 10⁻⁹ m)
E = 5.93 x 10⁻¹⁶ J
Therefore, the increment of energy (or quantum) that is emitted at 450nm by CuCl is 5.93 x 10⁻¹⁶ J.
To learn more about quantum
https://brainly.com/question/25786066
#SPJ1
What is the solubility of Mg(OH)₂ at a pH of 12.80? (Ksp Mg(OH)₂ is 1.6 × 10⁻¹³)
Answers
The calculations show that Mg(OH)₂ has a solubility of 3.5 × 10⁻⁵ M at a pH of 12.80.
The term pH, which stands for "potential of hydrogen ions," can be interpreted as a measurement of the molar concentration of hydrogen ions in a particular solution. Hence, the acidity, neutrality, or basicity of any chemical solution is often determined or specified using the power of hydrogen ions (pH).
Mg(OH)₂⇔Mg²⁺(aq)+2OH⁻(aq)
First of all, we would write the chemical equation for this chemical reaction that is appropriately balanced
The Ksp for the aforementioned chemical reaction is determined mathematically by:
Ksp = [Mg²⁺][OH⁻]²
Ksp = [x][2x]²
1.6 × 10⁻¹³ = 4x³
x = ∛4 × 10⁻¹⁴
x = 3.5 × 10⁻⁵ M.
The maximum amount of a chemical that will dissolve in a particular amount of solvent at a particular temperature is known as its solubility. Different compounds have very varying solubilities, which is a characteristic of a particular solute-solvent pair.
Learn more about solubility here
https://brainly.com/question/27278411
#SPJ1
What is the Heisenberg Uncertainty Principle?
A. We can know either the speed (momentum) or location, but not both at the same time.
B. We cannot ever know the speed (momentum) or location of the electron.
C. We can know only the speed (momentum) of the electron, but never the location.
D. We can know only the location of the electron, but never the speed (momentum).
Answers
Answer:
b option is correct
Explanation:
we cannot ever know the speed and location of the electron
Thermal energy depends on what two things?
Answers
Answer:
temperature and mass
Explanation:
porque
Hope this helps :)
Give the formula for the ionic compound formed from the gold(III) and chloride ions, an antimicrobial.
Answers
According to the given information, we are to find the formula for the ionic compound formed from the gold(III) and chloride ions.
Ionic compounds are compounds formed by ions. The cations and the anions are the ionic compounds formed generated by the ionic compounds. Cations are positively charged ions while anions are negatively charged ions.
• Cations - the ions formed by the ,loss of electrons.
,• Anions- the ions formed by the, gaining of electrons.
For the Gold(III) the formula for its ionic compound is as shown below:
\(\text{Gold(III)}=Au^{3+}\)Note that the chemical symbol for Gold is Au and the charge of +3 shows that the element loses 3 electrons in its outer shell to form a stable atom.
For chloride ion, the ionic compound is expressed as:
\(Chloride=Cl^-^{}\)The chemical symbol for Chlorine is Cl with a charge of -1. This shows that the element gains an electron to form a stable atom.
What volume will 2.91 moles of nitrogen occupy at 1.50 atm and 23°C?
Answers
A container with a volume of nitrogen gas is filled to 1.50 atm of pressure and 23°C of temperature. Compute the amount of gas in moles led with as at 2.91 moles is 18.87 liters.
The three gas laws that make up the combined gas law, also known as a general gas equation, are Charle's law, Boyle's law, and Gay-Lussac law. The law shows the link between temperature, volume, and pressure for a given amount of gas.
PR/T=V
V= 2.91×150/23
V=18.87liters
According to Boyle's Law, gas volume increases as pressure lowers. Charles' Law states that a gas expands as its temperature rises. Also, according to Avogadro's Law, a gas's volume increases as its concentration does.
The area occupied by a gas.The pressure a gas exerts against the container's walls.The actual temperature of the gas.Either the total amount of gaseous substance or the number of gas moles.
Learn more about moles here
https://brainly.com/question/15300621
#SPJ1
What is one advantage of asexual reproduction?
O A. It is advantageous in a changing environment.
B. It is the slowest way to reproduce.
O C. It results in genetically different offspring.
D. It requires only one parent.

Answers
Answer: D.
Explanation:
Asexual reproduction requires only one parent, making it more efficient and take a shorter amount of time than sexual reproduction.
A reaction produces 0.829 moles of H20. How many molecules of H2O are produced?

Answers
The molecules of H2O that are produced are: 4.98 × 10²³ molecules of H2O
What is meant by a reaction?Process in which one or more substances are converted to one or more different products is called a chemical reaction.
To calculate the number of molecules of H2O produced, we can use the Avogadro's number, which is equal to 6.022 × 10²³ molecules per mole of substance.
number of molecules = moles of substance × Avogadro's number
number of molecules = 0.829 moles × 6.022 × 10²³molecules/mol
number of molecules = 4.98 × 10²³molecules
Therefore, approximately 4.98 × 10²³ molecules of H2O are produced.
To know more about reaction, refer
https://brainly.com/question/11231920
#SPJ9
An atom with 14 protons, 14 neutrons, and 16 electrons is stable, -2 charge
stable, +2 charge
unstable, -2 charge
unstable, no charge *
Answers
We can see that an atom with 14 protons, 14 neutrons, and 16 electrons is unstable, and has a -2 charge.
So the correct option is the third one.
What can we say about the atom?An atom with 14 protons, 14 neutrons, and 16 electrons is not stable. The number of protons in an atom, also known as its atomic number, determines its element and its chemical properties. In this case, the atom has 14 protons, which corresponds to the element silicon (Si) on the periodic table.
For an atom to be stable, it should have a balanced number of protons and electrons. Electrons are negatively charged particles that orbit the nucleus of an atom in energy levels or electron shells. The number of electrons in a stable atom should be equal to the number of protons, resulting in a neutral charge overall.
In this case, the atom has 14 protons and 16 electrons, which means it has two more electrons than protons, resulting in a net charge of -2. This is an example of an ion.
Learn more about atoms.
https://brainly.com/question/17425565
#SPJ1
Which of the following is an example of quantitative data
Answers
Answer:
numerical values such as measurements, cost, and weight;
Explanation:
The hair colors of players on a football team
the color of cars in a parking lot
the letter grades of students in a classroom
the shape of candies in a variety pack
If d represents the density of a gas and k is a constant. The relationship between the rate of diffusion r, and d is ____?
Answers
The relationship between the rate of diffusion r, and d is r ∝ 1/√d.
The relationship between the rate of diffusion (r) and the density of a gas (d) can be explained using Graham's law of diffusion. According to this law, the rate of diffusion of a gas is inversely proportional to the square root of its density. Mathematically, it can be expressed as:
r ∝ 1/√d
where the symbol '∝' represents 'proportional to'. The constant of proportionality (k) can be introduced to this equation as:
r = k/√d
This equation shows that as the density of a gas increases, its rate of diffusion decreases. This is because denser gases have more molecules per unit volume and thus, they experience greater intermolecular collisions that hinder their movement. Therefore, it requires more energy for them to diffuse through a medium compared to less dense gases.
The relationship between the rate of diffusion and density is particularly important in understanding the behavior of gases in different environments. For instance, in a gas chromatography column, the rate of diffusion of a gas determines how quickly it moves through the column and separates from other components. Similarly, in the Earth's atmosphere, the rate of diffusion of greenhouse gases such as carbon dioxide affects their concentration and hence, their impact on climate change.
For more such questions on diffusion
https://brainly.com/question/29064792
#SPJ11
Which two types of weather are most likely to occur when you see clouds Like these?
__________________________________
◻ A. Sunshine
◻ B. Light rain
◻ C. Snow
◻ D. Thunderstorms
Final Warning⚠ Do Not Answer This Question If you do not no know The Answer!

Answers
Answer:
A: Sunshine
Explanation:
Snow and light rain.
I have did this quiz a while ago.
hope it will help you out. :)
What ways is carbon added to the atmosphere
Answers
Answer:
One way is through fossil fuels. When people burn fossil fuels it enters the atmosphere as carbon dioxide gas. Another way is through animals. Most animals exhale carbon dioxide as a waste product which gets into the atmosphere.
sketch an enthalpy diagram for the combustion of cyclopropane, 36().
36() +9/2 2 () → 32 () + 32 ()
° = −2091.3 /
Answers
The attached graphic displays the enthalpy diagram for the combustion of cyclopropane.
Does the reaction produce heat?The enthalpy diagram for a combustion reaction typically displays the change in reaction enthalpy (H) throughout the reaction route.
An enthalpy diagram normally has two sides: the reactant side and the product side. Reactant species like gasoline or hydrocarbons have a higher enthalpy on the reactant side when compared to the products. As the reaction gets nearer to the products, the enthalpy decreases.
Learn more about exothermic reaction:brainly.com/question/28546817
#SPJ1

How much table salt would dissolve in 540mL of water if the water was 25 degrees Celsius? ( please show work)
Answers
At 25 °C, the about 194.4 g of table salt would dissolve in 540 ml of water.
At 25 degrees, what is the water concentration?At 25 °C, pure water has a concentration of 55.5 M (mol/L). H+ and OH- ions are ionized to a minor extent. Electrical conductivity experiments show that the equilibrium constant, also known as the dissociation constant, is 1.8 10-16 M at 25.
How much sodium chloride dissolves in 100 mL of water at 100 degrees Celsius?We draw this conclusion from the fact that 39.2 grams of sodium chloride dissolve in 100 grams of water at 100 degrees Celsius. The result is that the solution has exceeded the limit of the maximum permitted amount of NaCl for that mass of water by it is characterized as a supersaturated solution because of the tiny margin.
To know more about concentration visit:-
https://brainly.com/question/10725862
#SPJ1
An Sulfur tetrafluoride gas is collected at 23.0 °C in an evacuated flask with a measured volume of 20.0 L. When all the gas has been collected, the pressure in the flask is measured to be 0.230 atm . Calculate the mass and number of moles of sulfur tetrafluoride gas that were collected. Be sure your answer has the correct number of significant digits. mass: 1 g N10 mole: mol X & ? Explanation Check
Answers
mass = 20.48 g
moles=0.1895
Further explanationIn general, the gas equation can be written
Pv=nRTwhere
P = pressure, atm
V = volume, liter
n = number of moles
R = gas constant = 0.08205 L.atm / mol K
T = temperature, Kelvin
P=0.23 atm
V=20 L
T=23+273=296 K
\(\tt n=\dfrac{PV}{RT}=\dfrac{0.23\times 20}{0.082\times 296}=0.1895\)
mass SF₄ (MW=108,07 g/mol) :
\(\tt 0.1895\times 108,07 g/mol=20.48~g\)
4. Diffuse reflection occurs when parallel light waves strike which surface?
Answers
Answer:
rough surface
Explanation:
Diffuse reflection occurs when parallel light waves strike a rough surface
Answer:
Rippling fountain
Explanation:
How do erosion and deposition work together to form sand dunes?
O Waves cause erosion along coastlines and deposit sand away from the shore,
Erosion occurs as surface water carries sediment and the sediment is deposited near oceans and lakes
O Glaciers cause erosion through the movement of large chunks of ice, which are deposited and form depressions
Erosion occurs through deflation, and sand that was picked up is deposited against an obstruction
Answers
Answer:
A
Explanation:
I took the test 100%, good luck
Erosion and deposition work together to form sand dunes as waves cause erosion along coastlines and deposit sand away from the shore. So the correct option is A.
What is erosion?Erosion is the removal of the material's surface from the crust of the Earth. It is mainly the debris of soil and rock. The eroded materials are transported by natural agents such as water or wind from the point of their removal.
The term erosion gives an explanation for the wearing down and molding of the landforms on the surface of Earth. This includes the rock weathering from its original position, the transport of this weathered material, and the erosion which is the result of the action of wind or glacial processes, etc.
The more appropriate term for this would be denudation or degradation. This includes processes of mass movement. This is a definition of erosion that is very narrow and excludes the process of the transport of eroded material by natural agencies.
Therefore, the correct option is A.
Read more about erosion, here
https://brainly.com/question/3852201
#SPJ6
The formation of ethyl alcohol (C2H5OH) by the fermentation of glucose (C6H12O6) may be represent by the following: C6H12O6 --> 2 C2H5OH 2 CO2 If a particular glucose fermentation process is 70.0% efficient, how many grams of glucose would be required for the production of 51.0 g of ethyl alcohol (C2H5OH)
Answers
Answer:
142.5 g
Explanation:
According to the chemical reaction:
C₆H₁₂O₆ --> 2 C₂H₅OH + 2 CO₂
1 mol of glucose (C₆H₁₂O₆) forms 2 moles of ethyl alcohol (C₂H₅OH) and 2 moles of carbon dioxide (CO₂).
We first convert the moles to grams by using the molecular weight (Mw) of each compound:
Mw (C₆H₁₂O₆) = (12 g/mol x 6) + (1 g/mol x 12) + (16 g/mol x 6)= 180 g/mol
1 mol C₆H₁₂O₆ = 180 g/mol x 1 mol = 180 g
Mw(C₂H₅OH) = (12 g/mol x 2) + (1 g/mol x 5) + 16 g/mol + 1 g/mol= 46 g/mol
2 mol C₂H₅OH = 2 mol x 46 g/mol = 92 g
Thus, when the process is 100% efficient, 180 grams of glucose produce 92 grams of ethyl alcohol. To form 51.0 grams of ethyl alcohol, we will need:
51.0 g C₂H₅OH x (180 g C₆H₁₂O₆/92 g C₂H₅OH) = 99.8 g C₆H₁₂O₆
As the process has a lower efficiency (70.0%), we will need more glucose to obtain the required yield. So, we divide the mass of glucose required for a process 100% efficient by the actual efficiency:
mass of glucose required = 99.8 g C₆H₁₂O₆/(70%) = 99.8 g C₆H₁₂O₆ x 100/70 = 142.5 g
Therefore, it would be required 142.5 grams of glucose to obtain 51.0 grams of ethyl alcohol.
How many grams of lead will be produced if 2.54g of PbS is burned with 1.88g of O2? write the equation
Answers
If 2.54 g of PbS is burned with 1.88 g of O2, approximately 2.20 grams of Pb will be produced.
The balanced equation for the reaction of lead sulfide (PbS) with oxygen (O2) to produce lead (Pb) and sulfur dioxide (SO2) is as follows:
2PbS + 3O2 -> 2Pb + 2SO2
From the balanced equation, we can see that the stoichiometric ratio between PbS and Pb is 2:2 or 1:1. This means that for every 1 mole of PbS, 1 mole of Pb is produced.
To calculate the number of moles of PbS, we need to divide the given mass (2.54 g) by its molar mass:
Molar mass of PbS = 207.2 g/mol (Pb) + 32.07 g/mol (S) = 239.27 g/mol
Moles of PbS = 2.54 g / 239.27 g/mol = 0.0106 mol
Since the stoichiometric ratio between PbS and Pb is 1:1, the number of moles of Pb produced is also 0.0106 mol.
To calculate the mass of Pb, we multiply the number of moles by its molar mass:
Molar mass of Pb = 207.2 g/mol
Mass of Pb = 0.0106 mol x 207.2 g/mol = 2.20 g
This calculation is based on the stoichiometric ratio between PbS and Pb, where 1 mole of PbS produces 1 mole of Pb. By converting the given mass of PbS to moles and then multiplying by the molar mass of Pb, we can determine the mass of Pb produced.
For more such question on PbS. visit :
https://brainly.com/question/27964828
#SPJ8
Which conditions produce the largest ocean waves?
Question 3 options:
strong winds that blow for a long time over a great distance
weak winds that blow for short periods of time with a short fetch
strong winds that blow for short periods of time with a short fetch
weak winds that blow for long periods of time with a long fetch
Answers
Answer:
Strong winds that blow for a long time over a great distance.
Explanation:
Generally, the biggest and most powerful wind-generated waves are produced by strong storms that blow for a sustained period over a large area. Huge and big waves, or swells, can travel over long distances. The size of the wave depends on wind speed, wind duration, and the area over which the wind is blowing. If The speed of the wind is more, it stays for along time and it covers a larger distance then the waves produced will be very powerful and large.