3. When two atoms bond together to form a molecule, which parts of the atoms
become part of the bond?
Answers
Answer:
Atoms come together to form molecules because of their electrons. Electrons can join (or bond) atoms together in two main ways. When two atoms share electrons between them, they are locked together (bonded) by that sharing. These are called covalent bonds
Related Questions
When discussing class data, Jack notices a trend: The difference between the experimental value for the specific heat and the accepted value for the 100% propylene glycol solution (pure propylene glycol) is higher than the others. Provide an explanation for why this might be true
Answers
According to the question the experimental value for the specific heat and the accepted value for the 100% propylene glycol solution (pure propylene glycol) is higher than the others as:
The experimental value is the value that you get in an experiment.
Error = [accepted value - experimental value]
Typically, the discrepancy is shown as a percent inaccuracy.
Experimental value 100% equals percentage error = |experimental value - accepted value. Consider, for instance, that you performed an experiment to ascertain the boiling point of water and obtained a result of 99.3 °C.
99.3 °C is your experimental value.
The ideal temperature is 100.0 °C.
|99.3 °C - 100.0 °C| = 0.7 °C is the experimental error.
The inaccuracy is represented as 99.3 °C - 100.0 °C/100.0 °C = 0.7 °C.
0.7/100.0 °C × 100 = 0.7 %
To know more about percentage error visit
https://brainly.com/question/28746643
#SPJ4
You order a glass of lemonade, 150 mL, in a restaurant only to discover that it is warm and too sweet. The sugar concentration of the lemonade is 2.27 M but you would like it to be reduced to a concentration of 1.88 M.How many grams of ice should you add to the lemonade, knowing that only a third of the ice will melt before you take your first sip? (Assume the density of water is 1.00 g/mL)
Answers
Since no sugar will be added or removed, the number or moles of it in the glas won't change.
Let's call this quantity:
\(n_s\)The molar concetrnation can be written as the followin equation:
\(C=\frac{n_s}{V}\)Where C is the concentration given the number of moles of sugar and the volume V.
We can rewrite this as:
\(n_s=C\cdot V\)Now, before the ice melts, the volume of the lemonate is 150 mL and the sugar concentration is 2.27 mol/L. Let's call this situation 1:
\(n_s=C_1\cdot V_1\)After the ice melt the one third it will, we will have a certain volume and the concentration we want 1.88 mol/L. Let's call this situation 2:
\(n_s=C_2\cdot V_2\)Now, we can put thouse tofether:
\(\begin{gathered} n_s=n_s \\ C_1\cdot V_1=C_2\cdot V_2 \end{gathered}\)And we can solve for the unknown volume of situation 2:
\(V_2=\frac{C_1\cdot V_1}{C_2}=\frac{2.27M\cdot150mL}{1.88M}=\frac{340.5}{1.88}mL=181.117\ldots mL\approx181mL\)Since the final volume is approximately 181 mL, the difference between it and the initial volume is the volume of water that came from the melted part of the ice:
\(181mL-150mL=31mL\)Since we assume that the density of the water is 1.00 g/mL, we can calculate the mass it represents:
\(\begin{gathered} \rho=\frac{m}{V}_{} \\ m=\rho\cdot V=1.00g/mL\cdot31mL=31g \end{gathered}\)And since this is only 1 third of the ice (the rest won;t melt), we know that the whole ice will have three times this mass:
\(m_{ice}=3\cdot31g=93g\)So, it should be added approximatelt 93 grams of ice.
I swear, everyone just ignores my question, but I'm going to ask anyways. A beaker is filled with 5.4 grams of Oxygen gas in a fixed volume of 4.0 liters. One of the valves opened and there remains only 1.7 grams of Oxygen gas, what is the new volume of gas?
Answers
Answer:
2 P2 (1.2 atm =157 one. 8. A gas with a volume of 4.0 L
Explanation:
because its central b atom has only 6 valence electrons, the species bf3 does not exists. true or false
Answers
False. The statement that the species BF3 does not exist because its central boron (B) atom has only 6 valence electrons .
The existence and stability of chemical species are determined by the electron configuration and bonding of the atoms involved. In the case of BF3 (boron trifluoride), boron is the central atom.
Boron, located in Group 13 of the periodic table, has an atomic number of 5. As a result, it has 5 electrons in its neutral state. However, when boron forms chemical compounds, it can utilize vacant orbitals to accommodate additional electrons.
In the case of BF3, boron forms three covalent bonds with three fluorine (F) atoms, resulting in a total of 8 electrons around the boron atom. This satisfies the octet rule, which states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with 8 valence electrons.
Therefore, BF3 does exist, and its central boron atom accommodates 8 valence electrons, rather than just 6.
Learn more about valence electrons here
https://brainly.com/question/12746595
#SPJ11
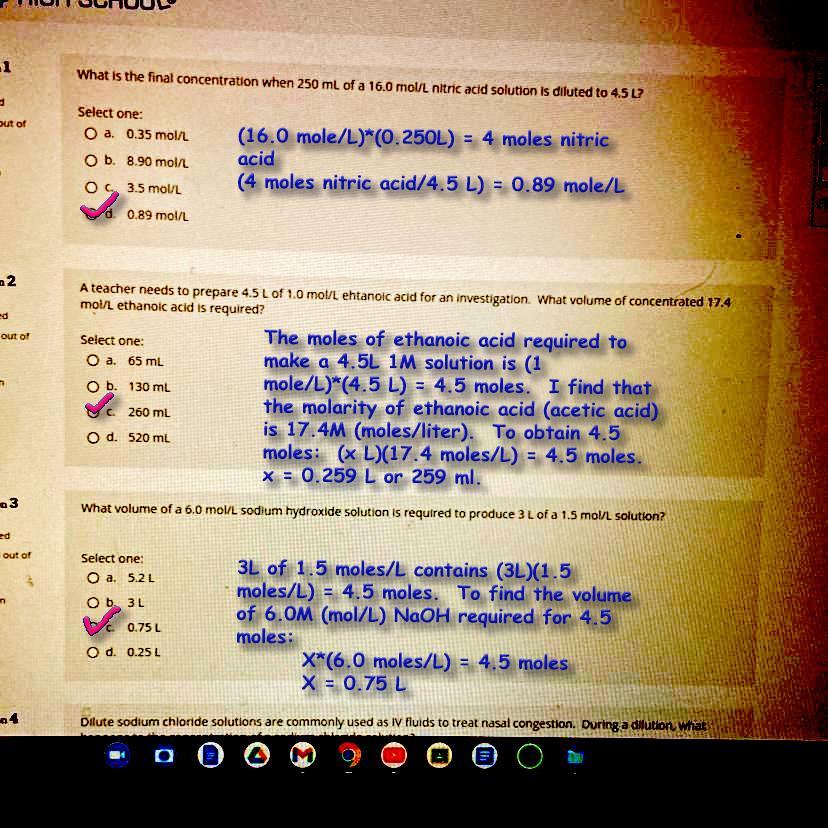
see the attached photo*** can someone please explain how to do this for me? I really need help on my summer school thank you !

Answers
Answer:
See attached image.
Explanation:
See attached image.
Remember to use the units in deciding steps to take. Find the moles needed or delivered by using:
Molarity x Volume = moles [(moles/L)*(L) = moles]
Molarity, M, is dedined as 1 mole/liter. When working with a unit such as 6M, rewite it as 6 moles/liter, and then use unit conversions to guide how to find the solution, so to speak.
Is state change physical or chemical? Explain how you know.
Answers
Answer:Physical
Explanation:
State Change is physical because it changes without it being chemically done. So when water changes from a liquid to a solid the state of it is changing. (Solid or Liquid or Gas)
Basically what is physically being changed with out a chemical.
In this lab you make a malachite solution and then dilute it, putting 1.00 mL of the concentrated solution into a 10.00 mL volumetric flask. You then measure the absorbance of the dilute solution and use that to determine concentration. If you determine the concentration of your diluted malachite solution to be 0.0112 M, what was the concentration of the concentrated solution? ___ M What calculation did you use to determine the concentration of the concentrated solution? a. Cc x Vc=Cd x Vd b. abs=(slope x conc) + y-intercept c. (mass of malachite/total mass of sample) x 100 d. ((actual - theoretical)/theoretical) x 100
Answers
The concentration of the concentrated solution was 0.112 M, and the calculation used was option a. Cc x Vc = Cd x Vd.
The concentration of the concentrated solution can be determined using the formula Cc x Vc = Cd x Vd. In this case, Cc is the concentration of the concentrated solution, Vc is the volume of the concentrated solution (1.00 mL), Cd is the concentration of the diluted solution (0.0112 M), and Vd is the volume of the diluted solution (10.00 mL).
To find Cc, rearrange the formula:
Cc = (Cd x Vd) / Vc
Plug in the values:
Cc = (0.0112 M x 10.00 mL) / 1.00 mL
Cc = 0.112 M
Learn more about diluted solution here:
https://brainly.com/question/1416865
#SPJ11
ammonium perchlorate is the solid rocket fuel that was used by the u.s. space shuttle and is used in the space launch system (sls) of the artemis rocket. it reacts with itself to produce nitrogen gas , chlorine gas , oxygen gas , water , and a great deal of energy. what mass of oxygen gas is produced by the reaction of 9.94 of ammonium perchlorate?
Answers
The mass of oxygen gas produced by the reaction of 9.94 g of ammonium perchlorate can be calculated using stoichiometry and the balanced equation for the reaction.
What is the balanced equation?
The balanced equation for the reaction of ammonium perchlorate (NH₄ClO₄) is:
NH₄ClO₄ → N₂(g) + Cl₂(g) + 2O₂(g) + 2H₂O(g)
From the balanced equation, we can see that for every 1 mole of NH₄ClO₄, 2 moles of O₂ are produced.
First, we need to determine the number of moles of NH₄ClO₄ in 9.94 g:
moles of NH₄ClO₄ = mass / molar mass = 9.94 g / (NH₄ClO₄ molar mass)
Next, we can use the mole ratio from the balanced equation to calculate the moles of O₂ produced:
moles of O₂ = moles of NH₄ClO₄ × (2 moles of O₂ / 1 mole of NH₄ClO₄)
Finally, we can convert the moles of O₂ to grams using the molar mass of O₂.
Therefore, the mass of oxygen gas produced can be calculated using the given information and stoichiometry.
To know more about mass of oxygen, refer here:
https://brainly.com/question/29610238
#SPJ4
H2 produced by the above reaction? Calculate the mass of NaCl required producing
35.5g of H2?
Answers
To produce 35.5g of H2, approximately 2055.49g of NaCl is required.
To calculate the mass of NaCl required to produce 35.5g of H2, we need to determine the stoichiometry of the reaction and use the molar mass of NaCl.
The balanced equation for the reaction is:
2NaCl + 2H2O -> 2NaOH + H2
From the balanced equation, we can see that for every 2 moles of NaCl, 1 mole of H2 is produced. We can use the molar mass of H2 (2.016g/mol) to convert the given mass of H2 into moles:
moles of H2 = mass of H2 / molar mass of H2
moles of H2 = 35.5g / 2.016g/mol
moles of H2 = 17.6 mol
Since the stoichiometry of the reaction is 2 moles of NaCl to 1 mole of H2, we can set up the following ratio:
moles of NaCl / moles of H2 = 2 / 1
Rearranging the equation to solve for moles of NaCl:
moles of NaCl = (moles of H2 * 2) / 1
moles of NaCl = (17.6 mol * 2) / 1
moles of NaCl = 35.2 mol
Now, we can calculate the mass of NaCl required using the molar mass of NaCl (58.44g/mol):
mass of NaCl = moles of NaCl * molar mass of NaCl
mass of NaCl = 35.2 mol * 58.44g/mol
mass of NaCl = 2055.49g
For more such questions on NaCl visit:
https://brainly.com/question/23269908
#SPJ8
The first-order rearrangement of ch3nc is measured to have a rate constant of 3. 61 x 10^-15 s-1 at 298 k and a rate constant of 8. 66 × 10^-7 s^-1 at 425 k. determine the activation energy for this reaction.
Answers
The activation energy for the first-order rearrangement of CH3NC is 1.6 x 10^5 J/mol, which can be determined using the Arrhenius equation. The equation relates the rate constant (k) to the temperature (T) and the activation energy (Ea).
The Arrhenius equation is given by: k = A * e^(-Ea/RT)
Where:
k = rate constant
A = pre-exponential factor
Ea = activation energy
R = gas constant
T = temperature
To determine the activation energy, we need to find the ratio of rate constants at two different temperatures and solve for Ea.
Taking the natural logarithm of both sides of the equation, we have:
ln(k2/k1) = -(Ea/R) * (1/T2 - 1/T1)
Given:
k1 = 3.61 x 10^-15 s^-1 at 298 K
k2 = 8.66 x 10^-7 s^-1 at 425 K
Plugging these values into the equation and solving for Ea:
ln(8.66 x 10^-7/3.61 x 10^-15) = -(Ea/R) * (1/425 - 1/298)
Ea = -ln(8.66 x 10^-7/3.61 x 10^-15) / (1/425 - 1/298) * R
Ea = -ln(2.4 x 10^8) / (0.00354) * 8.314
Ea = 1.6 x 10^5 J/mol
To determine the activation energy for the first-order rearrangement of CH3NC, we use the Arrhenius equation. This equation relates the rate constant (k) to the temperature (T) and the activation energy (Ea). By taking the natural logarithm of the ratio of rate constants at two different temperatures, we can solve for Ea. Given the rate constants at 298 K and 425 K, we plug these values into the equation and rearrange it to solve for Ea. Using the value of the gas constant R, we can calculate the activation energy.
learn mor about Arrhenius equation here: brainly.com/question/31887346
#SPJ11
Which part of the mantle is still a solid but flows like a thick, heavy liquid?
-lithosphere
-asthenosphere
-core
-crust
i will give brainest
Answers
Answer:
Asthenosphere
Explanation:
The asthenosphere is the hot, weak, inner part of the upper mantle. Although it is solid, it can flow like a thick, heavy liquid.
How mang millimoles of solute are contained in 2.00L of 2.76×10^-3 M of KMnO4
Answers
Answer:
5.52
Explanation:
2.76 10^-3 M = 2.76mM
2.76mM*2L= 5.52mmol
YOU ARE AMAZING AND YOU ARE SO IMPORTANT HAVE A GOOD DAY!
Answers
Answer:
Thx Have a fantastic day! :)
Explanation:
50 g of liquid water is frozen in an ice cube tray. What is the total weight of all of the ice cubes
Answers
Explanation: The water is still only going to be 50 grams. Although it’s in a different state, it didn’t gain or lose any water while being frozen.
Which is a stronger acid?
■ A) pH=4
■
B) pH=5
Answers
A pH of 4 has a higher concentration of H+ ions compared to option B with a pH of 5. Therefore, option A is a stronger acid.
pH is a measure of the concentration of hydrogen ions (H+) in a solution. Acids are substances that can donate H+ ions, and the strength of an acid depends on the concentration of H+ ions in solution. The lower the pH, the higher the concentration of H+ ions, and the stronger the acid. In this case, option A with a pH of 4 has a higher concentration of H+ ions compared to option B with a pH of 5. Therefore, option A is a stronger acid because it has a greater ability to donate H+ ions in solution compared to option B.
To learn more about Acids:
https://brainly.com/question/25148363
https://brainly.com/question/26855500
calculate the energy in hartrees (ha) of hydrogen in its second excited state h[0,0,1] where n = 3.
Answers
The energy in Ha for the second excited state of hydrogen (h[0,0,1]) is -1.51 Ha.
To calculate the energy of hydrogen in its second excited state, we need to use the formula for the energy of a hydrogen atom:
E = -13.6 ha / n^2
Where E is the energy in hartrees (ha), and n is the principal quantum number. In this case, n = 3, so we can plug that into the formula:
E = -13.6 ha / 3^2
E = -13.6 ha / 9
E = -1.51 ha
Therefore, the answer is E = -1.51 Ha.
To know more about excited state, refer here:
https://brainly.com/question/2289096#
#SPJ11
Baking soda has a pH of 8. Its a(n) _________ substance
Acid
Neutral
Alkaline
Powdery
(god bless!! have a wonderful and blessed day!!! i love you!! and dont forget no matter what god loves you too!!!
Answers
Answer:
it's an acid
Explanation:
because itis soda
What happens if an organism cannot carry out its life function?
Answers
Answer:
organisms cannot stay with out getting nutrients as they will not be able to repair them selves or produce energy.
Explanation:
When a change in a physical property is observed, Select the correct answer below: it could be because of a physical change. it must be because of a chemical change. it must be because of a chemical change and a physical change. it could be caused by a change that is considered both physical and chemical.
Answers
Answer:
It could be because of a physical change.
Explanation:
A physical change affects a substance's physical properties, and a chemical change affects its chemical properties.
during anaphase, the forces that drive movement of chromatids toward opposite spindle poles include which of the following?
Answers
All of the above provide the forces that drive the movement of chromatids toward opposite spindle poles during anaphase.
What is anaphase?
Anaphase is the third stage of cell division, which occurs between metaphase and telophase, and is characterized by movement. Following are the forces that drive the movement of chromatids toward opposite spindle poles.
Depolymerization of kinetochore microtubules at the plus end; directed kinesin motors operating on interpolar microtubules at the plus end, and aster microtubules being affected by minus-end-directed dynein motorsFor more questions like Anaphase click the link below:
https://brainly.com/question/13766396
#SPJ4
How many moles are in 5.67x10^24 atoms of RbCl?
Answers
Answer:
9.42 molesExplanation:
To find the number of moles in a substance given it's number of entities we use the formula
\(n = \frac{N}{L} \\\)
where n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
From the question we have
\(n = \frac{5.67 \times {10}^{24} }{6.02 \times {10}^{23} } \\ = 9.418604...\)
We have the final answer as
9.42 molesHope this helps you
the atomic number of an element x is 14 and it's relative atomic mass is 28
Answers
To get the number of neutrons use the formula i.e. No. of neutrons=mass number-atomic number. Therefore , 28–14 = 14neutrons.
Which element has a relative atomic mass of 28?Silicon-28 atom is the stable isotope of silicon with relative atomic mass 27.9769265.
How is relative mass determined?By multiplying the proton and neutron counts of the exact isotope that interests you, you may always find the relative mass of an element. For instance, an atom of carbon-12 has a relative atomic mass of 12 due to its composition of 6 protons and 6 neutrons.
To know more about Relative atomic mass visit:
https://brainly.com/question/25698972
#SPJ9
Why would you rather have hot cocoa than lemonade on a cold day? (The lesson is called heat transfer)
Answers
Answer:
you would more than likely have hot coca.
Explanation:
Because when its cold out you don't want something cold, its common sense lol.
HELPP please I’ll mark you as brainlister it’s 100 points

Answers
Answer:
4
Explanation:
hope this helps, can i have brainliest
2) How many moles of silicon are in a 56.18 g sample?
Answers
Draw the structure (s) - 1-bromo-1-chloropropane show wedges and dashes. Draw highest Newman projection looking down th C1-C2 bond
Answers
1-Bromo-1-chloropropane has a bromine atom bonded to the first carbon (C1), a chlorine atom bonded to the second carbon (C2), and the remaining carbons connected in a chain. The highest Newman projection looking down the C1-C2 bond shows the C1 atom in the front, the C2 atom at the back, and the other atoms (Br, C3, and Cl) attached to the C1 atom.
Here's the structure of 1-bromo-1-chloropropane, showing wedges and dashes:
Br
|
C
/
C
/
C - Cl
To draw the highest Newman projection looking down the C1-C2 bond, we need to imagine looking along that bond with the C1 atom in front and the C2 atom at the back. The attached atoms (Br, C1, C3, and Cl) will be represented as circles.
Here's the highest Newman projection:
Br
|
C3
/
C1
/
C2
/
Cl
The C1 atom is represented by the intersection of the horizontal and vertical lines, while the C2 atom is shown as the circle at the end of the vertical line. The other atoms (Br, C3, and Cl) are attached to the C1 atom, and their positions are represented by their corresponding circles.
To learn more about Newman projection,
https://brainly.com/question/30167216
#SPJ4
Would you expect soap to be a good rust remover? why or why not?.
Answers
Yes, actually dish soap (Dawn) and a potato can actually be good rust removers. Just by slicing a potato in half and then placing the cut end of the potato in a shallow dish of the dish soap, a process should be created after soaking for a few minutes. This is one of the DIY ways to remove rust; just remove the soapy potato from the shallow dish and take care of the rust. Simple as that meaning that dish soap would be a good rust remover.
~
ROR
The soap cannot be expected as a good rust remover, as it lacks the presence of strong acids, or petroleum products, to dissolve the rust.
What is rusting?Rusting is the process of the formation of iron compounds with the reaction of iron with oxygen and water.
The rust can be removed with the acid and petroleum products, as their reaction dissolves the oxygen compounds.
The soap alone cannot be considered a good rust remover, as it lacks the presence of strong acids or petroleum products to dissolve the rust.
Learn more about rusting, here:
https://brainly.com/question/688285
using the perioduc table determine the mass of one mole in the element carbon
Answers
One mole of carbon atoms has a mass of exactly 12 g. Because magnesium atoms each have twice the mass of carbon atoms (24Mg compared with 12C), one mole of magnesium has a mass of 24 g. In fact, one mole of any element has a mass in grams that is equal to its relative atomic mass. One mole of iron has a mass of 56 g
A scientist plans the react 9.0x10^-2 L NO held at a pressure of 200.0kPa and a temperature of 20.0 C with excess hydrogen. How many moles of nitrogen gas will be produced
Answers
Answer:
\(n_{N_2}=0.00369molN_2\)
Explanation:
Hello there!
In this case, by firstly setting up the described chemical reaction, we are able to write:
\(2NO(g)+2H_2(g)\rightarrow N_2(g)+2H_2O(g)\)
Thus, given the pressure (convert to atm), volume and temperature (convert to K) we can calculate the moles of NO:
\(n=\frac{PV}{RT}\\\\n=\frac{200.00/101.325atm*0.090L}{0.08206\frac{atm*L}{mol*K}*(20.0+273.15)K} \\\\n=0.00738molNO\)
Next, we use the 2:1 mole ratio of NO to N2 to obtain the moles of nitrogen gas that will be produced:
\(n_{N_2}=0.00738molNO *\frac{1molN_2}{2molNO} \\\\n_{N_2}=0.00369molN_2\)
Regards!
can anyone help me with this question?

Answers
If the weight in gm of the sample is 0.040 moles of salt (NaCl) then Molar mass of NaCl = 58.44 g/mol.
Define molar mass?The ratio between the weight and the amount of substance in any sample of a chemical compound is known as the molar mass of that compound. The molar mass of a compound is a bulk property instead of a molecular one.
By using formula: n=m / M m=n×M
m=(0.040)x(58.5) m=2.34g
So,
It means that if we prepare a 100cm3 solution using 2.34g of NaCl, the solution's concentration will be 0.500 mol/dm3.
Molar mass of Nacl = 58.44 g/mol
To know more about molar mass visit:
brainly.com/question/12127540
#SPJ1