2Ni(OH) → Ni2O + H2O
How many moles of water forms from 4.20 mol of nickel (I) hydroxide?
Answers
Answer:
2.1 moles of water formed.
Explanation:
Given data:
Moles of water formed = ?
Moles of Ni(OH) = 4.20 mol
Solution:
Chemical equation:
2Ni(OH) → Ni₂O + H₂O
Now we will compare the moles of Ni(OH) with water.
Ni(OH) : H₂O
2 : 1
4.20 : 1/2×4.20 = 2.1 mol
2.1 moles of water formed.
Related Questions
above what fe2 concentration will fe(oh)2 precipitate from a buffer solution that has a ph of 8.73 ?
Answers
Fe(OH)2 will precipitate from a buffer solution with a pH of 8.73 when the Fe2+ concentration exceeds 1.2 x 10-16.
The formation of Fe(OH)2 will occur when the Fe2+ concentration exceeds the solubility product of Fe(OH)2, which is Ksp = [Fe2+][OH-]2. At a pH of 8.73, [OH-] = 5.6 x 10-9.
Therefore, the solubility of Fe(OH)2 is Ksp = [Fe2+][5.6 x 10-9]2.
To calculate the Fe2+ concentration at which Fe(OH)2 will precipitate, we need to rearrange the equation to solve for [Fe2+].
\text{[Fe2+]} = \frac{\text{Ksp}}{\text{[OH-]}^2} = \frac{\text{Ksp}}{5.6 \times 10^{-9}}^2
Therefore, Fe(OH)2 will precipitate from a buffer solution with a pH of 8.73 when the Fe2+ concentration exceeds 1.2 x 10-16.
To know more about buffer solutions, refer here:
https://brainly.com/question/24262133#
#SPJ11
How many formula units are present in 4.00 moles of SrCl2?
Answers
Answer:
It took a while but i'm pretty sure its 1.3981717 units.
Explanation:
ASAP, Please show explanation, will give brainliest. Thank you.

Answers
The attraction between the magnesium and oxygen atoms is extremely strong because of the larger magnitude of their respective charges.
They have an extremely high lattice energy as a result, and it takes a lot of energy to break this lattice. As a result, MgO has a high melting point. Magnesium and oxygen atoms in magnesium oxide combine to create ions in the lattice. The attraction between the magnesium and oxygen atoms is extremely strong because of the larger magnitude of their respective charges. They have an extremely high lattice energy as a result, and it takes a lot of energy to break this lattice. As a result, MgO has a high melting point.
Learn more about magnesium here:
https://brainly.com/question/1533548
#SPJ1
Why does platinum metal make a good catalytic surface for reactant molecules?
It causes reactant molecules to be less reactive.
It strengthens bonds between reactant molecules.
It prevents products from breaking away from the surface.
It holds reactant molecules in a good position for them to react.
Answers
Answer:
it holds reactant molecules in a good position for then to react
Answer:
D. It holds reactant molecules in a good position for them to react.
Explanation:
Got a 100 on e2020.
when you touch a hot potato with your finger, energy flows
Answers
When you touch a hot potato with your finger, energy flows. Touch is a sense that is associated with the detection of pressure, temperature, and pain. In the scenario presented the touch of the hot potato by the finger results in the transfer of heat energy from the potato to the finger.
When the finger comes into contact with the hot potato, the energy from the potato flows to the finger. The hot potato has more energy, which is transferred to the finger, making the finger feel hotter. This heat energy transfer is caused by a difference in temperature between the two objects. The flow of energy from the hot potato to the finger occurs until both objects reach thermal equilibrium (the same temperature). In this case, the transfer of energy continues until the finger and the potato have reached the same temperature. The rate of energy transfer depends on the temperature difference between the two objects. The greater the temperature difference, the greater the rate of energy transfer.In conclusion, when you touch a hot potato with your finger, energy flows from the hot potato to the finger, resulting in the transfer of heat energy.
To know more about thermal equilibrium visit :
brainly.com/question/29419074
#SPJ11
If a lab group reports a percent yield of 90% for salt what is a possible explanation for the “missing” product
Answers
The missing product in a chemical reaction can be due to various reasons such as incomplete reaction, loss of product during isolation, errors in measurements, impurities in reactants or products, and side reactions.
Percent yield is a measure of the efficiency of a chemical reaction. It compares the actual yield of a reaction to the theoretical yield that could be obtained if the reaction went to completion. A percent yield of 90% means that 90% of the expected amount of product was obtained. The remaining 10% is the "missing" product.
The missing product can be due to several reasons. One possibility is that the reaction was incomplete, meaning that not all of the reactants were converted to products. This could happen if the reaction conditions were not optimal, such as if the temperature or pressure was not high enough or if the reaction time was too short.
Another possibility is that some of the product was lost during isolation, such as if it was stuck to the reaction vessel or if it evaporated. Errors in measurements can also contribute to the missing product, such as if the reactants were not accurately weighed or measured. Impurities in the reactants or products can interfere with the reaction, leading to a lower yield.
Finally, side reactions can also occur, where the reactants form other products instead of the desired product. This can happen if the reactants are not pure or if the reaction conditions are not optimal. Overall, identifying the reason for the missing product requires careful analysis of the reaction conditions and the experimental procedure.
learn more about percent yield here:
https://brainly.com/question/17042787
#SPJ9
How many elements, compounds and atoms are in CH3COOH
Answers
Match the landform to its description.
People Who Answer First And Got This Right, Will Be Mark As Brainilist!

Answers
Answer:
Cinder cone-made of pieces of lava
caldera-bowl-shaped depression
volcanic soil-rich in nutrients
lava plateau-material fills in valleys
shield volcano-wide summit, gentle slope
Explanation:
Just did it
Answer: See image
Explanation: match the landform to its description

name of the arrhenius acid that contains the fluoride anion
Answers
The name of the Arrhenius acid that contains the fluoride anion is hydrofluoric acid (HF). Hydrofluoric acid is a strong acid that consists of hydrogen (H) and fluoride (F) ions. In its pure form, hydrofluoric acid is a colorless liquid with a strong and pungent odor.
Hydrofluoric acid is unique because it is the only known inorganic acid that readily ionizes in water to produce fluoride ions (F⁻) and hydronium ions (H₃O⁺). The dissociation reaction can be represented as follows:
HF (aq) ↔ H⁺ (aq) + F⁻ (aq)
Fluoride anion (F⁻) is a highly reactive species and plays a crucial role in various chemical and biological processes. It is widely used in industrial applications, including the production of fluorine-containing compounds and as a reagent in organic synthesis.
However, hydrofluoric acid is also known for its hazardous properties. It is corrosive to the skin and can cause severe burns. Moreover, fluoride ions have the ability to penetrate tissues deeply and can lead to systemic toxicity. Therefore, the handling and use of hydrofluoric acid require proper safety precautions and protective measures.
To know more about Hydrofluoric acid, refer to the link below:
https://brainly.com/question/30750257#
#SPJ11
If you produce 10 g of water in the reaction from 20 g of O2 what is your percent yield? 2H2+O2>2H2O
Answers
Answer:
44.4%
Explanation:
The balanced chemical equation for the reaction is:
2 H₂(g) + O₂(g) → 2 H₂O(g)
According to the equation, 1 mol of O₂ produces 2 moles of water (H₂O). We convert the moles to grams by using the molecular weight (Mw) of each compound:
Mw(O₂) = 2 x 16 g/mol O = 32 g/mol
mass O₂ = 1 mol x 32 g/mol = 32 g O₂
Mw(H₂O) = (2 x 1 g/mol H) + 16 g/mol O = 18 g/mol
mass H₂O = 2 mol x 18 g/mol = 36 g H₂O
Thus, from 32 grams of O₂, 36 g of H₂O are produced (stoichiometric ratio= 36 g H₂O/32 g O₂). We calculate the amount of H₂O that would be produced from 20 grams of O₂ by multiplying the stoichiometric ratio by the mass of O₂, as follows:
36 g H₂O/32 g O₂ x 20 g O₂ = 22.5 g H₂O
The theoretical yield of H₂O is 22.5 g, and the actual amount produced is 10 g. So, we calculate the percent yield as follows:
Percent yield = actual yield/ theoretical yield x 100
= (10 g)/(22.5 g) x 100
= 44.4%
How would it have been if particles of matter had no space?
Answers
Answer:
Matter is made of small particles of atoms or molecules. ... The particles in the solid are touching with very little space between them. The particles in a liquid usually are still touching but there are some spaces between them. The gas particles have big distances between them.
a balloon contains 0.76 mol n2, 0.18 mol o2, 0.031 mol he and 0.026 mol at 739 mm hg. what is the partial pressure of o2?
Answers
Assume that every gas in the list will act perfectly. Using the mole fraction of O2 and the specified pressure, the partial pressure of O2 may be computed (P).
What is specified?
The following diagram illustrates the mathematical link between partial pressure of oxygen (P1) and oxygen mole fraction (X1):
P₁=X₁P
By dividing the total number of moles of gases (0.76 + 0.18 + 0.031 + 0.026) by the number of moles of O2 (0.18 mol), it is possible to determine the mole fraction of O2 as follows:
X₁= 0.18 / (0.76+0.18+0.031+0.026)= 0.1805
In this manner, the partial pressure of O2 (P1) is determined:
P1=0.1805x739mmHg, or 133.4mmHg
According to the estimate above, the partial pressure of oxygen is almost equal to 130 mm Hg. As a result, option C is the best one.
To learn more about specified visit
https://brainly.com/question/14137273
#SPJ4
9. A piece of iron has a volume of 11.6 cm and a mass of 63.5 g. What is its density?
O 75.1
9
cm3
O 0.18
9
cm3
O 5.74
9
cm3
O 51.9
Answers
Answer:
The answer is
5.474 cm³Explanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \\\)
From the question
mass = 63.5 g
volume = 11.6 cm³
The density is
\(density = \frac{63.5}{11.6} \\ = 5.4741379310...\)
We have the final answer as
5.474 cm³
Hope this helps you
did I do this right?

Answers
Look at the activity series and select which two of the following reactions would happen on their own. (Remember, if the lone element is more active than the metal in the compound, the lone element will react and replace the metal in the compound.) Lithium (Li)
Potassium (K)
Calcium (Ca)
Sodium (Na)
Aluminum (Al)
Zinc (Zn)
Iron (Fe)
Tin (Sn)
Lead (Pb)
(Hydrogen) (H)
Copper (Cu)
Silver (Ag)
Gold (Au)
A.
2Li + ZnBr2 ->2LiBr + Zn
B.
Al + 3LiCl ->AlCl3 + 3Li
C.
Sn + ZnSe ->SnSe + Zn
D.
3Ca + Al2O3 ->2Al + 3CaO
Answers
Answer:
2Li + ZnBr2 ->2LiBr + Zn
3Ca + Al2O3 ->2Al + 3CaO
Explanation:
Spontaneous reactions are reactions that can happen on their own. For a spontaneous reaction to occur, a metal that is higher in the activity series must displace a metal that is lower in the activity series from its solution and not vice versa.
If we look at the two reactions selected in the answer, lithium is above zinc in the activity series and calcium is above aluminum in the activity series hence the two reactions occur spontaneously.
Iron has a density of 7.9 g/cm3. What is the mass of a cube of iron with the length of one side equal to 55.0 mm?
Question 3 options:
1.3 × 103 g
2.3 × 10-2 g
4.3 × 102 g
2.1 × 104 g
1.4 g
Answers
The mass of the cube of iron with a side length of 55.0 mm is approximately 1313.6125 grams.
To calculate the mass of a cube of iron, we need to know the density of iron and the length of one side of the cube. Given that the density of iron is 7.9 g/cm^3 and the length of one side of the cube is 55.0 mm, we can proceed with the calculation. First, we need to convert the length of one side from millimeters (mm) to centimeters (cm) since the density is given in grams per cubic centimeter. We divide 55.0 mm by 10 to obtain 5.5 cm.
Next, we can calculate the volume of the cube using the formula V = (side length)^3. Substituting the value of 5.5 cm into the formula, we get V = (5.5 cm)^3 = 166.375 cm^3. Finally, we can calculate the mass of the cube using the formula mass = density × volume. Substituting the values of density (7.9 g/cm^3) and volume (166.375 cm^3), we get mass = 7.9 g/cm^3 × 166.375 cm^3 = 1313.6125 g.
In summary, to calculate the mass of the iron cube, we convert the length from millimeters to centimeters, calculate the volume of the cube, and then multiply it by the density of iron. The resulting mass is approximately 1313.6125 grams.
To learn more about formula mass click here:
brainly.com/question/28647347
#SPJ11
A topsoil has the following properties: Clay content: 30% Soil OM: 2%
CEC meq/100g: 15 pH: 4.8
A soil test lab recommended 4 tons/a of a 75% lime material. Estimate the final pH of the soil if all of this material were applied
Answers
The estimated final pH of the soil, if all 4 tons/a of the 75% lime material are applied, would be approximately 11.91. pH is a measure of the acidity or alkalinity of a solution. It is a logarithmic scale that ranges from 0 to 14, where a pH of 7 is considered neutral.
To estimate the final pH of the soil after applying the lime material, we need to consider the properties of the soil and the characteristics of the lime material. The lime material is 75% pure lime, which means it contains 75% calcium carbonate (CaCO3).
First, we calculate the lime requirement in pounds per acre (lbs/a):
Lime requirement (lbs/a) = Recommended lime rate (tons/a) * 2000
Given that the recommended lime rate is 4 tons/a, the lime requirement is:
Lime requirement (lbs/a) = 4 * 2000 = 8000 lbs/a
Next, we calculate the Effective Calcium Carbonate Equivalent (ECCE) for the lime material:
ECCE = % purity * CCE
CCE (Calcium Carbonate Equivalent) is a measure of the neutralizing ability of the lime material. Since the lime material is 75% pure lime, the ECCE is:
ECCE = 0.75 * 100 = 75%
Now, we calculate the lime index (LI):
LI = Lime requirement (lbs/a) / ECCE
LI = 8000 / 75 = 106.67 lbs/a
The lime index represents the amount of lime required to raise the pH of the soil by one unit. In this case, it is 106.67 lbs/a.
To estimate the final pH, we use the following equation:
Final pH = Initial pH + (LI / CEC)
Given that the initial pH of the soil is 4.8 and the CEC is 15 meq/100g, we can calculate the final pH:
Final pH = 4.8 + (106.67 / 15) = 4.8 + 7.11 = 11.91
The estimated final pH of the soil, if all 4 tons/a of the 75% lime material are applied, would be approximately 11.91.
To know more about pH, click here, https://brainly.com/question/31726972
#SPJ11
plzzz help me i promise will mark u brainiest

Answers
Answer:
d) water
Explanation:
Answer:
C) sodium chloride
when determining the standard reduction potential of a substance by using a standard hydrogen electrode as a reference, the standard reduction potential will always be equal to:
Answers
When determining the standard reduction potential of a substance by using a standard hydrogen electrode as a reference, the standard reduction potential will always be equal to overall cell potential.
The overall cell potential is the reduction potential of the substance being determined using the standard hydrogen electrode as a reference electrode since its electrode potential is set at zero volts.
What do you mean by overall cell potential?
The driving force of the electron flow from anode to cathode shows a potential drop in the energy of the electrons moving into the wire. The difference in potential energy between the anode and cathode is known as the cell potential in a voltaic cell.
To know more about overall cell potential from the given link:
https://brainly.com/question/4062394
#SPJ4
The standard reduction potential will always be equal to overall cell potential.
What is potential?
Potential is the capacity to become or develop into something in the future. It is the latent ability or capacity to achieve something great, or to realize a desirable outcome. Potential is the ability to produce a result, or to manifest something of value. It is associated with the idea of potentiality, which is the capacity to achieve something or to be something. Potential can refer to a wide range of situations, from physical and mental abilities to creative and professional potential. Potential is a concept that is often used to motivate people and challenge them to reach their highest level of performance. It can also be used to describe the potential of a given situation or environment, such as the potential of a business opportunity or a promising career path. Potential is an essential factor in personal development and growth, and it can be developed and nurtured over time.
To learn more about potential
https://brainly.com/question/17362810
#SPJ4
On base control is not recommended for alkaline perms since expansion is limited at the base and:a. chemicals weaken the curlb. causes a crease in the hairc. chemicals increase drynessd. tension may cause breakage
Answers
On base control is not recommended for alkaline perms since the expansion is limited at the base, which can lead to a variety of issues. One of the main concerns is that chemicals used in the process can weaken the curl and make it less defined over time.
This is especially true for alkaline perms, which use stronger chemicals than acid perms and can be more damaging to the hair.
Another issue with on base control for alkaline perms is that it can cause a crease in the hair. This is because the chemicals are applied directly to the base of the hair, which can cause it to bend or fold in an unnatural way. Additionally, the chemicals used in an alkaline perm can increase dryness in the hair, which can lead to breakage and other damage.
Finally, tension may cause breakage when using on base control for alkaline perms. This is because the hair is pulled tightly against the scalp, which can lead to breakage or damage if not done properly. Overall, it is recommended to avoid on base control for alkaline perms and instead use other techniques to achieve the desired curl and volume.
Learn more about desired curl here:
https://brainly.com/question/11612543
#SPJ11
A box contains 6 gas molecules. Microstates are distinguished by whether a given molecule is in the left or right half of the box. What percentage of time is the gas in the configuration where there are 3 molecules in each half of the box
Answers
The gas will spend approximately 12.5% of the time in the configuration where there are 3 molecules in each half of the box.
To calculate the percentage of time the gas is in the desired configuration, we need to determine the number of microstates that correspond to this configuration and compare it to the total number of possible microstates.
Let's denote the left half of the box as L and the right half as R. In the desired configuration, we have 3 molecules in L and 3 molecules in R.
The number of ways to choose 3 molecules out of 6 to be in L is given by the binomial coefficient, which can be calculated as:
C(6, 3) = 6! / (3! * (6-3)!)
C(6, 3)= 20
This means there are 20 different ways to arrange 3 molecules in the left half of the box.
Since the remaining 3 molecules must be in the right half, the total number of microstates corresponding to the desired configuration is also 20.
Now, let's determine the total number of possible microstates in the system.
Each molecule can independently occupy either L or R, resulting in 2 possibilities for each molecule. Since there are 6 molecules in total, the total number of microstates is:
2⁶ = 64
To find the percentage of time spent in the desired configuration, we divide the number of microstates corresponding to the desired configuration by the total number of microstates and multiply by 100:
(20 / 64) * 100 ≈ 31.25%
Therefore, the gas will spend approximately 31.25% of the time in the configuration where there are 3 molecules in each half of the box.
The gas will spend about 31.25% of the time in the configuration where there are 3 molecules in each half of the box.
To know more about gas visit:
https://brainly.com/question/6140407
#SPJ11
now calculate the theoretical percent hydrolysis for these 1m solutions. 1 M NaC2H3O2_______. 1 M Na2CO3_________.
Answers
To calculate the theoretical percent hydrolysis for the given 1 M solutions, we need to consider the hydrolysis reactions of the respective salts. Therefore, the theoretical percent hydrolysis for both 1 M NaC2H3O2 and 1 M Na2CO3 solutions is 100%.
For 1 M NaC2H3O2 (sodium acetate):
The hydrolysis reaction is as follows:
CH3CO2^- + H2O ⇌ CH3COOH + OH^-
Theoretical percent hydrolysis can be calculated using the equation:
Percent hydrolysis = [OH-] / initial concentration of salt × 100
Since NaC2H3O2 is a strong electrolyte, it completely ionizes in water, giving 1 M of CH3CO2^- ions.
Thus, [OH-] = 1 M
Percent hydrolysis = 1 M / 1 M × 100
= 100%
For 1 M Na2CO3 (sodium carbonate):
The hydrolysis reaction is as follows:
CO3^2- + 2H2O ⇌ HCO3^- + OH^-
Similar to the previous calculation, since Na2CO3 is a strong electrolyte, it completely ionizes in water, providing 1 M of CO3^2- ions.
Thus, [OH-] = 1 M
Percent hydrolysis = 1 M / 1 M × 100
= 100%
To know more about hydrolysis
https://brainly.com/question/30468294
#SPJ11
3. Determine the number of molecules of ethanol (C2H5OH) in 47.0g
Answer with explanation plsssss.
Answers
Answer:
6.14x10^24
Explanation:
convert the given 47.0g to mols then to molecules.
\(\frac{47.0g}{} x\frac{1mol}{46.07g}x\frac{6.022x10^{23} }{1 mol}\)
46.07 is the molar mass of ethanol
6.022x10^23 is avogadros number
Which is not an example of a compound?
Ingnore the top PLEASE HELPP
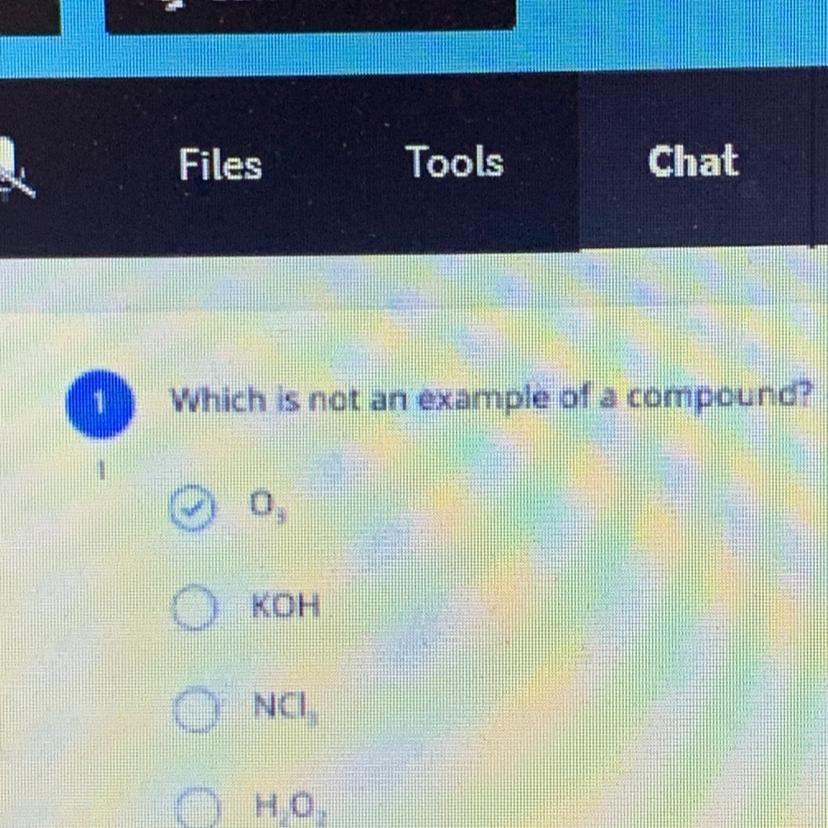
Answers
Answer:
The 3rd answer or NCI is the correct answer
Explanation:
Hope this helps:)
Answer:
the 1st one
Explanation:
Based on the parameters set forth in your lab procedure, what is the ph you should observe when you added the hcl to water?.
Answers
Acid has a pH below 7 while water has a pH of 7. A strong acid with a pH of roughly 3, HCl is. Water gets more acidic and loses pH in the range of 4-5 when HCl is added to it.
What is pH?A substance's pH is a gauge of how basic or acidic it is. It is a measurement of the amount of H+ present in the solution. It is equivalent to the negative logarithm of the concentration of H+ ions mathematically.
A solution is acidic if its pH value is less than 7, and basic if it is greater than 7. Acids have a lower pH because they contain more H+ ions. Strong acid hydrochloric acid has a pH between 2 and 3.
Water has no charge. Water becomes acidic in pH if any acid is introduced. Therefore, if HCl is given to water, the pH of the water will change to 3-5 depending on the acid content.
To find more on pH refer here:
brainly.com/question/491373
#SPJ4
use the values of ∆hof given below to calculate ∆horxn for the following reaction: 2no(g) o2(g) → 2no2(g) given: ∆hof (kj/mol) no(g) 90 o2(g) 0 no2(g) 34
Answers
The standard enthalpy of reaction (ΔH°rxn) for the reaction 2NO(g) + O₂(g) → 2NO₂(g) is is -112 kJ/mol.
To calculate the ΔH°rxn for the reaction 2NO(g) + O₂(g) → 2NO₂(g) using the given ΔH°f values, you can use the following equation:
ΔH°rxn = ΣΔH°f(products) - ΣΔH°f(reactants)
Given the ΔH°f values:
NO(g) = 90 kJ/mol
O₂(g) = 0 kJ/mol
NO₂(g) = 34 kJ/mol
Now, plug in the values:
ΔH°rxn = [2 × ΔH°f(NO₂)] - [2 × ΔH°f(NO) + ΔH°f(O₂)]
ΔH°rxn = [2 × 34] - [2 × 90 + 0]
ΔH°rxn = 68 - 180
ΔH°rxn = -112 kJ/mol
Thus, the ΔH°rxn for the reaction is -112 kJ/mol.
Learn more about standard enthalpy of reaction: https://brainly.com/question/28303513
#SPJ11
To what extent are countries adapting to the effects of climate change, and why is adapting more challenging for some countries than others?
Answers
Answer:
Progress made in Asia and the Middle East is clear, with nations such as China, Singapore and Malaysia all demonstrating increased adaptability. Much of Africa has also made progress since 1995, along with South American nations, including Brazil and Chile.
compare the size of ions to the size of atoms from which they form
Answers
Cations are always smaller than the atoms from which they form. Anions are always larger than the atoms from which they form. Ions are usually bigger than the atoms from which they are formed.
When an atom receives or loses electrons, the atom's electron configuration changes, resulting in a net positive or negative charge.
This net charge expands the electron cloud surrounding the nucleus, making the ion bigger in size than the neutral atoms from which it arose. When a metal atom loses one or more electrons to create a cation, it shrinks in size because the positive charge of the nucleus pulls the remaining electrons more strongly.
When a nonmetal atom obtains one or more electrons to create an anion, it normally expands in size.Because of the increasing amount of electrons, the electron cloud surrounding the nucleus grows. It should be noted that this comparison is not absolute and is dependent on the individual factors involved. Some ions are smaller than their neutral atom counterparts, while others are similar in size.
learn more about atoms here:
https://brainly.com/question/29695801
#SPJ4
The complete question is:
Compare the size of ions to the size of atoms from which they form.
Calculate the number of atoms in 24.83g calcium phosphate.
Answers
Avogadro's Number:
Avogadro's number, which is equal to 6.02214076 x \(10^{23}\) , is the quantity of units in one mole of any material. Hence, 1 mole of calcium phosphate,\(Ca_{3} (PO_{4}) _{2}\) will contain 6.02214076 x \(10^{23}\) atoms.
The molar mass of calcium phosphate is 310.18 g/mol,
Then, number of moles in 24.83g of calcium phosphate = \(\frac{mass}{molar mass}\)
= \(\frac{24.83}{310.18}\)
= 0.08 mole
Then, 0.08 mole of \(Ca_{3} (PO_{4}) _{2}\) will contain,
= 0.08 moles x 6.02214076 x \(10^{23}\) atoms
= 0.4818 x \(10^{23}\) atoms = 4.818 x \(10^{22}\) atoms.
Hence, 0.08 mole of calcium phosphate will contain 4.818 x \(10^{22}\) atoms.
To know more about Avogadro's number, visit,
https://brainly.com/question/2292441
#Avogadro's number
The free energy released by the hydrolysis of ATP under standard conditions is -30.5 kj/mol
. If ATP is hydrolyzed under standard conditions except at is more or less free energy released? Explain."
. If ATP is hydrolyzed under standard conditions except at is more or less free energy released? Explain.
Answers
If ATP is hydrolyzed under standard conditions except that pH is less than the standard pH, the free energy released will be less than -30.5 kJ/mol. This is because the concentration of H+ ions is greater than the standard pH, and this can affect the ionization of the phosphate groups in ATP.
ATP is hydrolyzed under standard conditions except that pH is less than the standard pH:If ATP is hydrolyzed under standard conditions except at pH less than the standard pH, it means the pH of the solution is more acidic than the standard pH. In this case, the concentration of H+ is greater than the standard pH, and this can affect the ionization of the phosphate groups in ATP. Because the phosphate groups have pKa values of around 6 and 7, the concentration of H+ ions can affect the protonation of the phosphate groups in ATP.
In this scenario, the free energy released by the hydrolysis of ATP will be less than -30.5 kJ/mol because the reaction is not taking place under standard conditions. Therefore, if ATP is hydrolyzed under standard conditions except that pH is less than the standard pH, less free energy will be released. This is because the reaction is not occurring under standard conditions and therefore the standard free energy change does not apply.
Under standard conditions, the free energy released by the hydrolysis of ATP is -30.5 kj/mol. However, if ATP is hydrolyzed under standard conditions except that pH is less than the standard pH, the free energy released will be less than -30.5 kJ/mol. This is because the concentration of H+ ions is greater than the standard pH, and this can affect the ionization of the phosphate groups in ATP. Because the phosphate groups have pKa values of around 6 and 7, the concentration of H+ ions can affect the protonation of the phosphate groups in ATP. As a result, the reaction will not take place under standard conditions and therefore the standard free energy change does not apply.
To know more about ATP, refer
https://brainly.com/question/897553
#SPJ11