Answers
Answer
d. Change of state
Explanation
a. Change in color is a sign of a chemical change.
b. Gas is given off is a sign of a chemical change.
c. Light is given off is a sign of a chemical change.
d. Change of state is not a sign of a chemical change.
Related Questions
2.
Which mixture could be a useful buffer in a solution?
acetic acid (CH3CO2H) and hydrochloric acid (HCl)
sodium hydroxide (NaOH) and elemental sodium (Na)
ammonia (NH3) and ammonium chloride (NH4Cl)
acetic acid (CH3CO2H) and ammonia (NH3)
Pls answer quickly
Answers
Ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)) mixture could be a useful buffer in a solution. Option C
A buffer is a solution that can resist changes in pH when small amounts of acid or base are added. It consists of a weak acid and its conjugate base or a weak base and its conjugate acid. The buffer system works by the principle of Le Chatelier's principle, where the equilibrium is shifted to counteract the changes caused by the addition of an acid or a base.
In option A, acetic acid (\(CH_3CO_2H\)) is a weak acid, but hydrochloric acid (HCl) is a strong acid. This combination does not form a buffer because HCl is completely dissociated in water and cannot provide a significant concentration of its conjugate base.
Option B consists of sodium hydroxide (NaOH), which is a strong base, and elemental sodium (Na), which is a metal. This combination does not form a buffer as there is no weak acid-base pair involved.
Option D contains acetic acid (\(CH_3CO_2H\)), a weak acid, and ammonia (\(NH_3\)), a weak base. Although they are weak acid and base, they do not form a buffer system together as they are both weak acids or bases and lack the required conjugate acid-base pair.
Option C, ammonia (\(NH_3\)), is a weak base, and ammonium chloride (\(NH_4Cl\)) is its conjugate acid. This combination can form a buffer system. When ammonia reacts with water, it forms ammonium ions (NH4+) and hydroxide ions (OH-).
The ammonium ions act as the weak acid, while the ammonia acts as the weak base. The addition of a small amount of acid will be counteracted by the ammonium ions, and the addition of a small amount of base will be counteracted by the ammonia, thus maintaining the pH of the solution relatively stable.
Therefore, option C, consisting of ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)), is the suitable mixture that could be a useful buffer in a solution.
For more such question on buffer visit:
https://brainly.com/question/13076037
#SPJ8
What are all the possible metabolites for the clofibrate structure through metabolism pathways like hydrolysis, alkylation, conjugation, oxidation etc. ?
Answers
Clofibrate is a lipid-lowering pharmaceutical that has been utilized to treat hyperlipidemia. Its metabolism system includes different pathways such as hydrolysis, oxidation, conjugation, and others.
What are a few potential metabolites of clofibrate?Metabolites are little molecules that are produced during the process of metabolism in living living beings.
Clofibrate hydrolysis metabolite:
2-(4-Chlorophenoxy)-2-methylpropanoic acid (phenoxyisobutyric acid)Clofibrate oxidation metabolites:
4-Chlorophenylacetic acid4-Chlorobenzaldehyde4-Chlorophenylacetyl-CoAClofibrate alkylation metabolites:
No specific alkylation metabolites have been widely reported for clofibrate.Clofibrate conjugation metabolites:
Glucuronide conjugatesSulfate conjugatesConclusively, the relative abundance and significance of each metabolite can vary among individuals.
Learn more about metabolites
https://brainly.com/question/14422941
#SPJ1
prop-1-yne + 2HBr/H2O2 = A;
A + 2H2O = B;
B + K2CO3(aq) = C;
C + heat = D;
D + HBr = E.
find the compounds A, B, C, D and E
Answers
Based on the given reactions, the compounds are as follows:
A: The specific product formed from the reaction between prop-1-yne and either 2HBr or H2O2.
B: The product formed when compound A reacts with 2H2O.
C: The product formed when compound B reacts with K2CO3(aq).
D: The product formed from the heat-induced reaction of compound C.
E: The product formed when compound D reacts with HBr.
Based on the given reactions, let's analyze the compounds involved:
Reaction 1: prop-1-yne + 2HBr/H2O2 = A
The reactant prop-1-yne reacts with either 2HBr or H2O2 to form compound A. The specific product formed will depend on the reaction conditions.
Reaction 2: A + 2H2O = B
Compound A reacts with 2H2O (water) to form compound B.
Reaction 3: B + K2CO3(aq) = C
Compound B reacts with K2CO3(aq) (potassium carbonate dissolved in water) to form compound C.
Reaction 4: C + heat = D
Compound C undergoes a heat-induced reaction to form compound D.
Reaction 5: D + HBr = E
Compound D reacts with HBr (hydrobromic acid) to form compound E.
For more such questions on compounds
https://brainly.com/question/704297
#SPJ8
J.J. Thomson discovered the electron in 1897. In 1904, he proposed a model of an atom, describing that there was an equal distribution of negative and positive charges throughout the atom. In 1909, Ernest Rutherford tested J.J. Thomson’s model by shooting positive particles at gold foil. Based on Thomson's model, it was predicted that the particles would fly through the foil with a small amount deflected back. Analyzing the results, Rutherford discovered that more of the particles bounced back than expected. Which of the following best explains how the results of Rutherford’s experiment affected Thomson’s widely-accepted atomic model?
A. Rutherford’s results were invalidated and discarded because Thomson’s model was correct.
B. Rutherford’s results supported parts of Thomson's model, but also provided new data and interpretations.
C. Rutherford’s results suggested that the model proposed by Thomson was based on false research and required a change in his hypothesis.
D. Rutherford’s results supported Thomson’s model that there was a negative core surrounded by positive charges and caused a modification in the overall atomic theory.
Answers
Rutherford's results declined the results of Thomson's model, the correct option is C.
What are Atomic models?Atomic Models are the scientific theories proposed to determine the structure of an atom.
There are mainly 5 theories proposed for atomic models.
1. John Dalton's Atomic Model: An atom is the basic building block of all physical entities in the universe.
2. Plum Pudding Model, created by J.J. Thomson, uses the comparison of plum pudding, where the positive charge is uniformly dispersed throughout and the negative charge is randomly sprinkled on top, to explain how subatomic particles are structured.
3. Rutherford's model: proved the presence of a nucleus.
4.Niel Bohr's model: Arrangement of electrons in shells.
5. Erwin Schrodinger's model is also called as Quantum Model.
In J.J. Thomson's model, the equal distribution of positive and negative charges is proposed while,
Rutherford's theory declines this arrangement and proposes that the positively charged nucleus occupies a very small part and there is empty space in the atom.
So, Rutherford’s results suggested that the model proposed by Thomson was based on false research and required.
To know more about Atomic Models
https://brainly.com/question/9145431
#SPJ6
True or false? Increasing the force will increase the moment.
Answers
The statement "increasing the force will increase the moment" is true.
This is because the moment is a measure of the turning effect of a force on an object about a pivot point. It is defined as the product of the force and the perpendicular distance between the force and the pivot point. The unit of moment is the newton-meter (Nm) or the joule (J).When a force is applied to an object, it will produce a moment about the pivot point if the force is not acting along the same line as the pivot point. The magnitude of the moment depends on the force applied and the distance of the force from the pivot point. As the force increases, the moment also increases, provided that the distance from the pivot point remains constant. Conversely, if the force remains constant, but the distance from the pivot point increases, the moment also increases. This is because the perpendicular distance is directly proportional to the moment, meaning that a longer distance results in a larger moment.Therefore, it can be concluded that increasing the force applied to an object will increase the moment produced about a pivot point.
for such more questions on force
https://brainly.com/question/24719118
#SPJ8
how many grams of Fe are produced from 92.5g of FeO given the following reaction
Answers
The mass(in grams) of iron, Fe produced from the 92.5g of iron (ii) oxide, FeO is 71.9 grams
How do i determine the mass of Fe produced?The mass of Fe produced from the 92.5g of iron (ii) oxide, FeO can be obtained as follow:
2FeO → 2Fe + O₂
Molar mass of FeO = 71.85 g/molMass of NH₃ from the balanced equation = 2 × 71.85 = 143.7 g Molar mass of Fe = 55.85 g/molMass of Fe from the balanced equation = 2 × 55.85 = 111.7 gFrom the balanced equation above,
143.7 g of FeO reacted to produce 111.7 g of Fe
Therefore,
92.5 g of Fe will react to produce = (92.5 × 111.7) / 143.7 = 71.9 g of Fe
Thus, the mass of Fe produced from the reaction is 71.9 grams
Learn more about mass produced:
https://brainly.com/question/9526265
#SPJ1
Complete question:
How many grams of Fe are produced from 92.5 g of FeO given the following reaction 2FeO → 2Fe + O₂
Draw an example of a buffer in the body.
Answers
Explanation:
this is answer.

In a molecule of calcium sulfide, calcium has two valence electron bonds, and a sulfur atom has six valence electrons. How many lone pairs of electrons are present in the Lewis structure of calcium sulfide?
A. one
B. two
C. three
D. four
E. none
Answers
Answer:
E.none
Explanation:
because the bonds join with each other and no lone pair is left
80 POINTS
Someone pls help me out

Answers
2) The heat capacity of aluminum is 219.44 J/mol.°C.
3) a) the experimental ΔHs of ice is -0.154 kJ/mol.
b) too high
How to calculate heat capacity?Calculate the heat released by the aluminum:
q = mcΔT
where q = heat released, m = mass of aluminum, c = specific heat capacity of water and ΔT = change in temperature.
q = (24.7 g) (0.903 J/g°C) (100.0°C - 23.4°C)
q = 18643.26 J
Next, calculate the heat absorbed by the calorimeter:
q = mcΔT
q = (99.5 g + 24.7 g) (15.8 J/°C) (23.4°C - 19.5°C)
q = 4009.92 J
The heat released by the aluminum is equal to the heat absorbed by the calorimeter and water:
18643.26 J = 4009.92 J + q3
where q3 = heat absorbed by the water.
q3 = 14633.34 J
Calculate the molar heat capacity of aluminum:
Cp,m = q3 / (nΔT)
where Cp,m = molar heat capacity, n = number of moles of aluminum, and ΔT = change in temperature.
n = m / M
where m = mass of aluminum and M = molar mass of aluminum (26.98 g/mol).
n = 24.7 g / 26.98 g/mol
n = 0.916 mol
Cp,m = 14633.34 J / (0.916 mol * 76.6°C)
Cp,m = 219.44 J/mol.°C
Therefore, the heat capacity of aluminum is 219.44 J/mol.°C.
3) (a) To calculate the experimental ΔHs of ice, we first need to calculate the heat gained by the water and the heat lost by the ice during the process.
Heat gained by water = mass of water × specific heat capacity of water × change in temperature
= 100.0 g × 4.184 J/g·°C × (-20.1°C)
= -8,423.84 J
Heat lost by ice = mass of ice × heat of fusion of ice
= 25.6 g × 6.01 kJ/mol
= 154.496 J
Since the process is assumed to be adiabatic (no heat exchange with the surroundings), the heat gained by the water must be equal to the heat lost by the ice.
Thus, -8,423.84 J = 154.496 J = -8,269.344 J
The negative sign indicates that the process is exothermic. Therefore, the experimental ΔHs of ice is:
ΔHs = -154.496 J/mol = -0.154 kJ/mol
(b) If the student forgets to include the calorimeter term in the calculation, the calculated ΔHs of ice will be too high. This is because the heat absorbed by the calorimeter during the process is not accounted for, leading to an overestimation of the heat gained by the water and underestimation of the heat lost by the ice.
Find out more on heat capacity here: https://brainly.com/question/16559442
#SPJ1
According to Boyle’s law, when the pressure of a gas increases at constant temperature, its volume
Answers
Answer:
the volume increases
Explanation:
How many electrons would be too many for a nitrogen atom
Answers
write an apology letter to your principal telling him why you were not in the mathematics competition.in 4000 words
Answers
Nitrogen gas reacts with hydrogen gas to produce ammonia gas .
Write a balanced chemical equation for this reaction.
Answers
Answer:
hope it helps
Explanation:
The balanced chemical equation for the formation of ammonia gas by the reaction between nitrogen gas an hydrogen gas is given. N2+3H→2NH3.
If 500.0 kJ of energy is released in the synthesis of water from its elements, how many grams of water are formed?
O2(g) + 2H2(g) → 2H₂O(l) + 572 kJ
Answers
15.6 grams of water are formed when 500.0 kJ of energy is released in the synthesis of water from its elements.
Grams of water calculation.
The balanced chemical equation for the synthesis of water from its elements is:
O2(g) + 2H2(g) → 2H₂O(l) + 572 kJ
The given energy change is -500.0 kJ, which means that energy is released during the reaction.
We can use the relationship between the amount of energy released and the amount of substance involved in the reaction to calculate the mass of water formed.
The molar enthalpy change for the reaction can be calculated as follows:
ΔH = -500.0 kJ/mol H2O
The molar enthalpy change tells us how much energy is released when 1 mole of water is formed.
The molar mass of water (H2O) is 18.015 g/mol.
To calculate the mass of water formed, we can use the following equation:
mass of water = (ΔH / molar enthalpy change) x molar mass of water
mass of water = (-500.0 kJ / mol H2O / -572 kJ/mol) x 18.015 g/mol
mass of water = 15.6 g
Therefore, 15.6 grams of water are formed when 500.0 kJ of energy is released in the synthesis of water from its elements.
Learn more about grams of water below.
https://brainly.com/question/24258132
#SPJ1
You want to test how the mass of a reactant affects the speed of a reaction.
Which of the following is an example of a controlled experiment to test this?
O A. The mass of all the reactants is varied, and the time it takes the
reaction to finish is measured.
B. The mass of one reactant and the temperature of the reaction
mixture are increased until the reaction is finished.
C. The mass of one reactant at a time is varied, and the time it takes
the reaction to finish is measured.
D. The mass of all of the reactants is kept the same, and the mixtures
are allowed to react for different lengths of time.
Answers
Para saken
Sorry po kung mali
A nearby pond has what appears to be steam coming off of it after a cold front passes through. What is it?
a. evaporation
b. sublimation
c. vaporization
d. condensation
Answers
Answer:
a. evaporation .
Explanation:
In this case, given the described situation, we should take into account that there are two types of liquid-gas phase transitions, evaporation and vaporization, which occur in totally different way.
Firstly, evaporation is a superficial phenomena, it means that it occurs at the surface of the liquid only whereas the vaporization is a bulk phenomena, which means that it occurs along the whole volume of liquid.
In such a way, we can infer that cold steam stream flowing over the pond has the capacity to strip or remove liquid water molecules in the pond and take them to the vapor phase, which means that the answer is a. evaporation .
Best regards.
The composition of a compound is 28.73% K, 1.48% H, 22.76% P, and 47.03% O. The molar mass of the
compound is 136.1 g/mol.
I
Answers
The compound has an empirical formula of \(K_2H_2P_2O_8\) and a molecular formula of \(K_2HPO_4\).
The given compound has a percent composition of K = 28.73%, H = 1.48%, P = 22.76%, and O = 47.03%. Its molar mass is 136.1 g/mol. To determine its molecular formula, we need to find its empirical formula and calculate its molecular formula from its empirical formula.The empirical formula is the smallest whole number ratio of atoms in a compound. It can be determined by converting the percent composition of the elements into their respective moles and dividing each by the smallest number of moles calculated. The moles of K, H, P, and O in 100 g of the compound are: K = 28.73 g x (1 mol/39.1 g) = 0.734 molH = 1.48 g x (1 mol/1.01 g) = 1.46 molP = 22.76 g x (1 mol/30.97 g) = 0.736 molO = 47.03 g x (1 mol/16.00 g) = 2.94 molDividing each by the smallest number of moles gives the following ratios: K = 0.734/0.734 = 1H = 1.46/0.734 = 2P = 0.736/0.734 = 1.002O = 2.94/0.734 = 4. The empirical formula of the compound is \(K_2H_2P_2O_8\). To calculate the molecular formula, we need to determine the factor by which the empirical formula should be multiplied to obtain the molecular formula. This can be done by comparing the molar mass of the empirical formula to the molar mass of the compound.The molar mass of \(K_2H_2P_2O_8\) is: \(M(K_2H_2P_2O_8)\) = (2 x 39.1 g/mol) + (2 x 1.01 g/mol) + (2 x 30.97 g/mol) + (8 x 16.00 g/mol) = 276.2 g/mol. The factor by which the empirical formula should be multiplied is: M(molecular formula)/M(empirical formula) = 136.1 g/mol/276.2 g/mol = 0.4935. The molecular formula is obtained by multiplying the empirical formula by this factor: \(K_2H_2P_2O_8 * 0.4935 = K_2HPO_4\). Therefore, the molecular formula of the compound is \(K_2HPO_4\).The molecular formula of the given compound having a composition of 28.73% K, 1.48% H, 22.76% P, and 47.03% O with a molar mass of 136.1 g/mol is \(K_2HPO_4\). The empirical formula of the compound is \(K_2H_2P_2O_8\). The compound's molecular formula is calculated by determining the factor by which the empirical formula should be multiplied to obtain the molecular formula. The factor is M(molecular formula)/M(empirical formula) = 136.1 g/mol/276.2 g/mol = 0.4935. The molecular formula of the compound is obtained by multiplying the empirical formula by this factor, resulting in the molecular formula \(K_2HPO_4\).For more questions on empirical formula
https://brainly.com/question/13058832
#SPJ8
The correct question would be as
The composition of a compound is 28.73% K. 1.48% H, 22.76% P, and 47.03% O. The molar mass of the compound is 136.1 g/mol. What is the Molecular Formula of the compound?
\(KH_2PO_4\\KH_3PO_4\\K_2H_4P_20_{12}\\K_2H_3PO_6\)
Stephan’s mother cuts a twig from a rose bush and plants it in the soil. After a few days, Stephan observes a new plant growing. Which characteristic does the growth of the new plant depict?
Answers
The growth of the new plant depicts the asexual reproduction characteristic. The characteristic that describes the growth of the new plant in Stephan's mother cutting a twig from a rose bush and planting it in the soil is asexual reproduction.
Asexual reproduction is the mode of reproduction by which organisms generate offspring that are identical to the parent's without the fusion of gametes. Asexual reproduction is a type of reproduction in which the offspring is produced from a single parent.
The offspring created are clones of the parent plant, meaning they are identical to the parent.The new plant in Stephan’s mother cutting a twig from a rose bush and planting it in the soil depicts the process of asexual reproduction, which is the ability of a plant to reproduce without seeds. In asexual reproduction, plants can reproduce vegetatively by cloning themselves using their roots, bulbs, or stems.
Know more about characteristic here:
https://brainly.com/question/28790299
#SPJ8
CuI2 (light brown solid) name copper compounds
Answers
CuI2 is not a known compound. Copper compounds typically have different oxidation states for copper, resulting in various compound names.
Copper(II) oxide (CuO): It is a black solid compound where copper is in the +2 oxidation state. It is commonly used as a pigment and in catalytic reactions.
Copper(II) sulfate (CuSO4): It is a blue crystalline compound in which copper is in the +2 oxidation state. It is used in various applications such as agriculture, electroplating, and as a laboratory reagent.
Copper(I) oxide (Cu2O): It is a red crystalline compound in which copper is in the +1 oxidation state. It is used as a pigment, in solar cells, and as a catalyst.
Copper(II) chloride (CuCl2): It is a greenish-brown solid compound in which copper is in the +2 oxidation state. It is utilized in various chemical processes, including etching and catalyst synthesis.
Copper(II) nitrate (Cu(NO3)2): It is a blue crystalline compound where copper is in the +2 oxidation state. It is commonly used in the production of catalysts, as a coloring agent, and in electroplating.
These are just a few examples of copper compounds with different oxidation states and properties. It's important to note that the compound CuI2 mentioned in the question, if it exists, would be an exception to the typical nomenclature for copper compounds.
For more such questions on oxidation visit:
https://brainly.com/question/13182308
#SPJ8
Which of the following is a correctly written thermochemical equation?
2C8H18 +25O2 → 16CO2 + 18H2O, ΔH = –5,471 kJ/mol
Correct Answer
C5H12 (g) + 8O2 (g) → 5CO2 (g) + 6H2O (l), ΔH = –3,536.1 kJ/mol
Incorrect Response
C3H8 (g) + O2 (g) → CO2 (g) + H2O (l), ΔH = –2,220 kJ/mol

Answers
Answer:
The second option
Explanation:
C5H12 (g) + 8O2 (g) → 5CO2 (g) + 6H2O (l), ΔH = –3,536.1 kJ/mol
Explanation:
The correctly written thermochemical equation should include the balanced chemical equation and the corresponding enthalpy change (ΔH). The balanced chemical equation must have equal numbers of atoms on both sides of the equation, and the state of each substance should be indicated. The enthalpy change should be written with the correct sign (positive or negative) and unit (kJ/mol).
The first equation is correctly balanced, but the enthalpy change is not correct. The correct enthalpy change for this reaction is –5,471 kJ/mol, but it is written as a positive value in the equation. The correct enthalpy change should be written as ΔH = –5,471 kJ/mol.
The second equation is correctly balanced and includes the correct enthalpy change (ΔH = –3,536.1 kJ/mol). Therefore, it is the correctly written thermochemical equation
Convert 2.40 x 10 23 molecules of an anonymous substance with a molar mass of 18.02 g/mol to its mass in grams.
Answers
The mass of 2.40 × 10²³ molecules of anonymous substance with molar mass of 18.02g/mol is 7.18grams.
How to calculate mass?The mass of a substance can be calculated by multiplying the number of moles in the substance by its molar mass as follows:
mass = no of moles × molar mass
First, we convert the number of molecules in the anonymous substance to moles as follows:
2.40 × 10²³ ÷ 6.02 × 10²³ = 0.39moles
mass of anonymous substance = 0.39 moles × 18.02g/mol = 7.18grams
Learn more about mass at: https://brainly.com/question/13320535
#SPJ1
Concentration (mol dm-³) 0.5- 0.4- 0.3- 0.2- 0.1 2. 3 5 The following equilibrium reaction is given: 2HI(g) = H₂(g) + I₂(g) Time (s) H₂/ HI Cy A change in pressure will not affect equilibrium in this case as the number of moles of gas is the same on both sides of the equation. AH> 0 A graph plotting the concentrations of the substances present versus time is given in Figure 7.10. a) b) Explain the physical situation in the container from t=0 s to t = 5 s. Which external factor was altered in order to bring about a change in the shape of the graph at t = 5 s? Explain. Calculate Kat t = 3 s. 1 dm³ COCI, decomposes
Answers
Based on the information provided, we have a reaction between hydrogen iodide (HI) gas and hydrogen gas (H₂) to form iodine gas (I₂). The equilibrium is represented by the equation:
2HI(g) = H₂(g) + I₂(g)
The concentration values given in the table correspond to the concentrations of H₂ and HI at different times.
a) From t=0 s to t=5 s: Without the specific graph mentioned in Figure 7.10, it is difficult to provide a precise explanation of the physical situation in the container during this time period. However, based on the equilibrium reaction given, we can make some general observations. At the start (t=0 s), the concentrations of H₂ and HI may be high. As time progresses, the reaction proceeds, and the concentrations of H₂ and HI may decrease while the concentration of I₂ increases. The specific behavior will depend on the rate of the forward and reverse reactions.
b) External factor altered at t=5 s: To bring about a change in the shape of the graph at t=5 s, some external factor must have been altered. The most likely factor is the total pressure within the container. Since the reaction involves gases, changes in pressure can affect the equilibrium position. However, according to the information given, a change in pressure will not affect equilibrium in this case since the number of moles of gas is the same on both sides of the equation. Therefore, if the shape of the graph changes at t=5 s, some other external factor, such as temperature or the addition of a catalyst, must have been altered.
c) Calculation of K at t=3 s: The equilibrium constant (K) can be calculated at any given time using the concentrations of the reactants and products. However, the concentrations of H₂ and HI at t=3 s are not provided in the information given. Without the necessary data, it is not possible to calculate K at t=3 s.
Lastly, the statement "1 dm³ COCI, decomposes" seems incomplete. If you provide additional information or clarify the question, I'll be happy to assist you further.
See attached. Thank you very much!!! :)





Answers
The functional groups that are present the compound is shown in the image is;
HaloalkaneAlkeneAmineWhat is the functional group?The functional group is the atom, group of atoms or bond that is responsible for the chemical reactivity of the members of a particular family of organic compounds. We have to note that there are several families of organic compounds and the reactivity of these families is determined mostly by the kind of functional group that we have in that particular molecule.
Now we have the structure that has been shown in the image attached. We now have to look at the image and determine the kind of functional groups that we have in the molecule. In this case, we are actually looking out for the very points along the chain where a chemical reaction is possible.
We can see that the functional groups that we have here are;
HaloalkaneAlkeneAmineLearn more about functional group:https://brainly.com/question/14618322
#SPJ1
What is the compound name for MgCO ?
Answers
What is the percent of C in Ca(C2H3O2)2?
(Ca = 40.08 g/mol, C = 12.01 g/mol, H= 1.01 g/mol, O= 16.00 g/mol)
[?]% C
Answers
The percent by mass of the carbon is 30.4%.
What is the percentage of calcium?The term percentage has to do with the ratio of the mass of a particular atom to the total mass of the compound multiplied by one hundred. Thus the first step is to find the total mass or the molar mass of the compound.
Molar mass = 40 + 2(2(12) + 3(1) + 2(16))
= 40 + 2(24 + 3 + 32)
= 40 + 2(59)
= 40 +118
= 158
Thus the mass of carbon is;
4(12)/158 * 100/1
= 30.4%
Thus carbon is only about 30.4% by mass of the compound.
Learn more about percent by mass:https://brainly.com/question/5394922
#SPJ1
Choose which of the following are organic. (check all that apply) *
Carbohydrates
Lipids
COH
NaOH
O
Proteins
Answers
Answer:
The answer is A, B and E
Explanation:
A - carbohydrates
B - Lipids
C- COH
E - Proteins
all except O and NaOH
Why should precision volumetric glassware be rinsed with distilled water after use?
Answers
Answer:
it should be rinsed to clean the glassware
You come across the following container while working in the lab: Answer the following questions in the space below: 1. Identify the WHMIS symbols. 2. What precautions should you take and why?
Answers
Type #1 Flame symbols are among the WHMIS emblems.
Type 2: Symbols with a flame above a circle.
Exploding bomb symbols are of type 3.
Compressed gas symbols are of type 4.
Corrosion symbols are type #5.
Skull and water the water symbols are type #6.
Exclamation mark symbols are type #7.
Health hazard symbols are type #8.
Because workplaces require a defined technique to detect hazardous items, WHMIS labels are crucial.
What does the WHMIS stand for?The national ’s hazard standard for Canada is the Health And Safety At work System (WHMIS). Hazard categorization, cautionary container labeling, the distribution of safety data sheets, and worker information and training programs are the system's main components.
What does WHMIS look like in the US?The U.S. Ohs Hazard Identification Standard and WHMIS are quite similar.
To know more about WHMIS visit:
https://brainly.com/question/28542158
#SPJ1
Classify each of the following word equations as a synthesis, decomposition, single displacement, or double displacement reaction.
Will give brainliest.
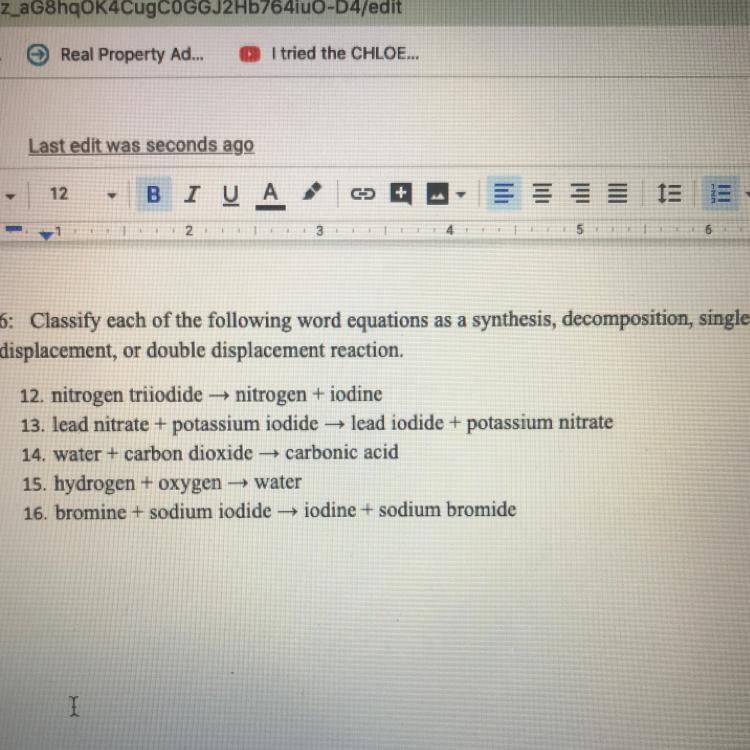
Answers
Answer:
12: This is decomposition because nitrogen triiodide is breaking apart.
13: This is double displacement because the elements in the compounds are "switching".
14: This is synthesis because the water and carbon dioxide are combining.
15: This is also synthesis because the hydrogen and oxygen are combining.
16: This is single displacement because the sodium is "switching" the element it's bonding with.
What the mechanisms of action of acidic acid with asprin
Answers
Synthesis. The synthesis of aspirin is classified as an esterification reaction. Salicylic acid is treated with acetic anhydride, an acid derivative, causing a chemical reaction that turns salicylic acid's hydroxyl group into an ester group (R-OH → R-OCOCH3).
hope it's help
#carryONlearning