Answers
Answer:
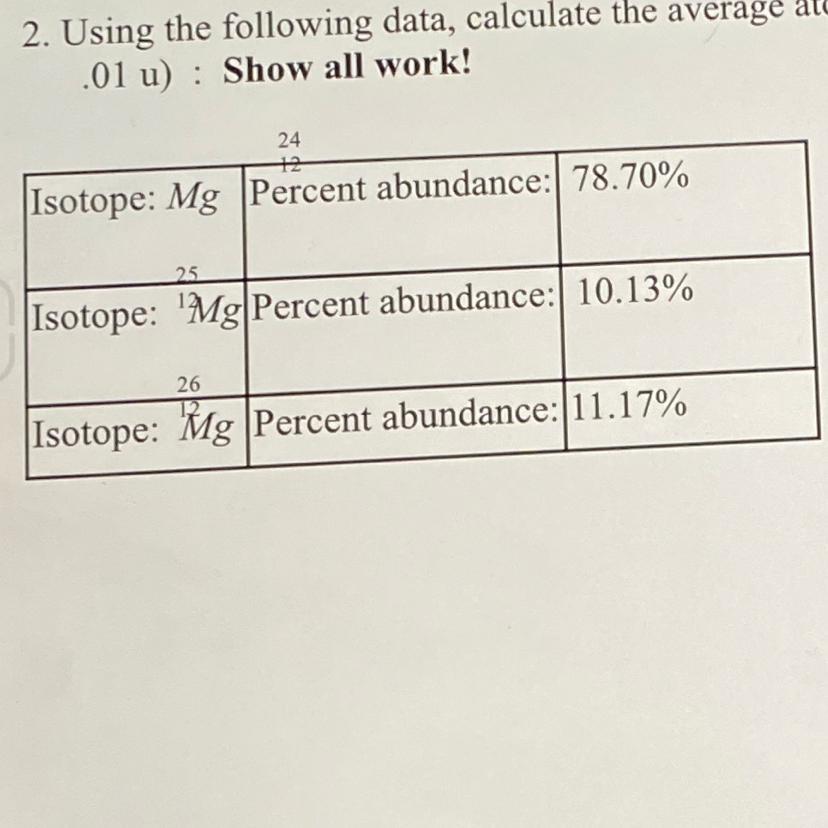
24.32
Explanation:
From the question given above, the following data were obtained:
Isotope A:
Mass of A = 24
Abundance (A%) = 78.70%
Isotope B
Mass of B = 25
Abundance (B%) = 10.13%
Isotope C:
Mass of C = 26
Abundance (C%) = 11.17%
Average atomic mass of Mg =..?
The average atomic mass of Mg can be obtained as illustrated below:
Average atomic mass = [(Mass of A × A%)/100] + [(Mass of B × B%)/100] + [(Mass of C × C%)/100]
Average atomic mass = [(24 × 78.70)/100] + [(25 × 10.13)/100] + [(26 × 11.17)/100]
= 18.888 + 2.5325 + 2.9042
= 24.3247 ≈ 24.32
Therefore, the average atomic mass of magnesium (Mg) is 24.32
Related Questions
1. In a heterogeneous mixture, the substances
A. can be physically separated.
B. are chemically combined.
C. can only be separated through chemical
processes.
D. break down into new substances.
Please answer
Answers
Heterogeneous mixtures is a mixture of two or more chemical substances (elements or compounds), where the different components can be visually distinguished and easily separated by physical means.
Hope this helps!
Brainliest and a like is much appreciated!
What is the concentration in mol L-l of a 12% solution of tetrahydrate magnesium chloride (MgCl2)?
Mg = 24.31 g mol-1
Cl = 35.45 g mol-1
H = 1.01 g mol-1
O = 16.00 g mol-1
Answers
The concentration in mol L-l (M) = 0.717
Further explanationGiven
12% solution of tetrahydrate magnesium chloride (MgCl₂)
Required
The concentration
Solution
Tetrahydrate magnesium chloride (MgCl₂)⇒MgCl₂.4H₂O
MW = Ar Mg+2. Ar Cl+8. Ar H + 4. Ar O
MW=24.31 + 2 x 35.45 + 8 x 1.01 + 4 x 16
MW=24.31+70.9+8.08+64
MW=167.29 g/mol
12% solution = 12 % m/v = 12 g in 100 ml solution
For 1 L solution :
\(\tt \dfrac{1}{0.1}\times 12~g=120~g\)
The concentration in g/L = 120 g/L
Convert grams to moles :
\(\tt mol=\dfrac{120}{167.29}=0.717\)
Given the following reaction: 2N₂O(g) + O₂(g) 4NO(g), predict the effect of each of the followingstressors (circle one)a. Increasing the pressure shift left shift right no change b. Removing O₂ shift left shift right no change C. Adding NO shift left shift right no changeD. Adding N2O shift left shift right no changeE. Addition of a catalyst shift left shift right no change
Answers
Answer:
a. Shift left.
b. Shift left.
c. Shift left.
d. Shift right.
e. No change,
Explanation:
Let's remember Le Chatelier's Principle: Le Chatelier's principle states that if a dynamic equilibrium is disturbed by changing conditions, the position of equilibrium shifts to counteract the change to re-establish an equilibrium.
Let's write the reaction:
\(2N_2O+O_2\leftrightarrows4NO.\)Now, let's analyze each statement:
a. We can apply pressure because all species in the reaction are in the gas phase. Remember that increasing the pressure on a gas reaction shifts the position of equilibrium towards the side with fewer moles of gas molecules. You can see in the reaction that on the left side we have 3 moles in total, but on the right side, we have 4 moles in total, so the equilibrium goes to the left. Shift left.
b. If we reduce the concentration of a substance in a reaction, in this case, O2 as the reactant, we will not increase the concentration of the products, so the equilibrium goes to the left. Shift left.
c. If we add NO, we will increase the concentration of N2O and O2, so in this case, the equilibrium goes to the left. Shift left.
d. This situation is similar to the last, but, we are on the other side. Increasing the concentration of N2O will increase the concentration of NO, so the equilibrium goes to the right. Shift right.
e. Remember that adding a catalyst makes absolutely no difference to the position of equilibrium, and Le Châtelier's principle does not apply. Based on this logic, there's no change.
You are eating a pizza. What type of mixture are you consuming?
Answers
Answer:
heterogeneous mixture
Explanation:
because pizza has different phases I think
The type of mixture that pizza can be classified is Heterogeneous mixture
Heterogeneous mixture can be regarded as a type of mixture that has it's composition not uniform throughout the entire mixture.Heterogeneous mixture is usually comprised of two or more , instance of this is combination of oil and water.A pizza can be considered as heterogeneous mixture, this is because it doesn't appear as non-uniform substance, it's composition the same at all point in the mixture.The components that made of pizza can be separated by use of physical means.Therefore, heterogeneous mixture can be regarded as one having more than a substance.
Learn more at : https://brainly.com/question/5139963?referrer=searchResults
5. Aspirin (acetylsalicylic acid, HAsp, Mr-180.2) is absorbed from stomach with the form of free
acid. A patient takes some antacid to adjust the pH of gastric juice to 2.95, then takes0.65 g of aspirin.
Assume aspirin dissolves immediately, and the pH of gastric juice is invariant. What is the mass of aspirin that the patient can absorb from stomach at once? pK, of acetylsalicylic acid is 3.48.
CAnswer m= 0.50
Answers
The patient's stomach can absorb 0.50 g of aspirin at once.
How to determine mass?The equation for the dissociation of acetylsalicylic acid is:
HAsp ⇌ H+ + Asp-
The dissociation constant (Ka) can be calculated from the pKa value as:
Ka = 10^(-pKa)
Ka = 10^(-3.48) = 2.51 x 10⁻⁴
At pH 2.95, the concentration of H+ ions can be calculated from the equation:
pH = -log[H+]
[H+] = 10^(-pH) = 1.13 x 10⁻³ M
The concentration of Asp- ions can be calculated from the equation:
Ka = [H+][Asp-]/[HAsp]
[Asp-] = Ka[HAsp]/[H+]
[Asp-] = (2.51 x 10⁻⁴)(0.65 g / 180.2 g/mol) / (1.13 x 10⁻³ M)
[Asp-] = 0.0036 M
The total amount of aspirin that can be absorbed is the sum of the amounts of HAsp and Asp-:
m = (0.65 g / 180.2 g/mol) + (0.0036 M)(0.1 L)(180.2 g/mol)
m = 0.50 g
Therefore, the patient can absorb 0.50 g of aspirin from the stomach at once.
Find out more on aspirin here: https://brainly.com/question/25794846
#SPJ1
An Arrhenius acid releases what ion when dissolved in water?Group of answer choicesOH- (hydroxyl ion)H+ (hydrogen ion)Na+ (sodium ion)H2O (water)
Answers
An arrhenius acid is an acid that dissociates in water to form hydrogen ions (H + ).
Example :
\(H_2SO_4(aq)\leftrightarrows\text{ H}^+(aq)\text{ + HSO}_4^-_(aq)\text{ }\)Dissociation of an arrhenius acid that yields H+ ion .{extra notes , on thearrhenius base dissociate in water to form OH- ions }
How can constraints be used to help define the problem?
Answers
Answer: Constraints are restrictions (limitations, boundaries) that need to be placed upon variables used in equations that model real-world situations. It is possible that certain solutions which make an equation true mathematically, may not make any sense in the context of a real-world word problem.
Explanation:
Constraints is a condition which helps in optimization that solution satisfies.
What are constraints?Constraints are logical conditions that solution to a problem of optimization must satisfy.In defining constraint, value of interest is computed using variables of decision.
There are 5 types of constraints:
1) NOT NULL constraints- They prevent null values to be entered.
2) unique constraints-ensures that each value is different from others and is not null.
3) Check constraints-It is a database rule specifying values in one or more columns.
4) foreign key constraints- It gives definition of required relationships between and within tables.
5) informative constraints-It is a constraint which is used by the SQL compiler for improving access to the data.
Learn more about constraints here:
https://brainly.com/question/15148586
#SPJ2
Help me please omg I don’t know

Answers
Answer:
5 1 2 4and 3 this is correct way
Help pls! Which of the following shows a balanced nuclear reaction?
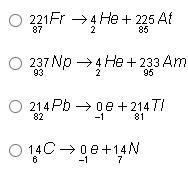
Answers
Answer:
Option 4: ¹⁴₆C —> ⁰₋₁e + ¹⁴₇N
Explanation:
To know which option is correct, do the following:
Option 1:
²²¹₈₇Fr —> ⁴₂He + ²²⁵₈₅At
87 = 2 + 85
87 = 87
221 = 4 + 225
221 ≠ 229
Thus the equation is not balanced.
Option 2:
²³⁷₉₃Np —> ⁴₂He + ²³³₉₅Am
93 = 2 + 95
93 ≠ 97
237 = 4 + 233
237 = 237
Thus, the equation is not balanced.
Option 3:
²¹⁴₈₂Pb —> ⁰₋₁e + ²¹⁴₈₁Tl
82 = –1 + 81
82 ≠ 80
214 = 0 + 214
214 = 214
Thus, the equation is not balanced.
Option 4:
¹⁴₆C —> ⁰₋₁e + ¹⁴₇N
6 = –1 + 7
6 = 6
14 = 0 + 14
14 = 14
Thus, the equation is balanced.
From the illustrations above, only option 4 is correct.
Consider the following reaction:
2CH4(g)⇌C2H2(g)+3H2(g)
The reaction of CH4 is carried out at some temperature with an initial concentration of [CH4]=0.092M. At equilibrium, the concentration of H2 is 0.014 M.
Find the equilibrium constant at this temperature.
Answers
The equilibrium constant at this temperature is Kc= 4.17 x 10⁻⁶.
What is equilibrium?Since the equilibrium constant depends on the equilibrium concentration of both the reactants and the products of the chemical reaction.
Balanced reaction equation
2CH₄(g)⇌C₂H₂(g)+3H₂(g)
The initial concentration of the CH₄ = 0.093 M
The equilibrium concentration of the H = 0.017 M
Equilibrium constant = ?
Let's make the ice table
2CH₄(g) ⇌ C₂H₂(g) + 3H₂(g)
0.093 M 0 0
-2x +x +3x
0.093-2x x 0.017 M
3x = 0.017 M
Therefore, x =0.017 M /3 = 0.00567 M
Therefore, the equilibrium concentration of CH₄ =
0.093 M – 2x = 0.093 M – (2 x 0.00567 M) = 0.0817 M
Equilibrium concentration of the C₂H₄ = x = 0.00567 M
Let's write the equilibrium constant expression
Kc= [C₂H₄[H2]³/[CH₄]²
Let's put the values in the formula
Kc= [0.00567][0.017]³/[0.0817]²
Kc= 4.17 x 10⁻⁶
Therefore, the equilibrium constant is 4.17 x 10⁻⁶.
To learn more about equilibrium constant, refer to the link:
https://brainly.com/question/12971169
#SPJ9
How many people were considered undernourished in Latin America and the Caribbean in 2018?
Question 4 options:
513.9 million
2.6 million
821.6 million
42.5 million

Answers
So let’s say that u just ate some hot chips and then u ate chicken and macaroni

Answers
Answer: oml
Explanation: thats gross
i'd eat the pic below then that

Are coins worth more because of their size?
Answers
Answer:
nope
Explanation:
they are not based on size because dimes are the smallest coin but is worth 10 cents and a penny is bigger then a dime but is worth only 1 cent
How would you indicate two molecules of sucrose?
Answers
Answer:
Sucrose is a disaccharide; each molecule consists of two "simple" sugars (a glucose and a fructose), called monosaccharides.
Which best describes the energy change that takes place during deposition?
Heat energy released by the substance
Heat energy is maintained by the substance
Heat energy is slowly gained by the substance
Heat energy is quickly absorbed by the substance
Answers
Answer:
heat energy released by the substance
The energy change that takes place during deposition is that heat energy is released by the substance. This statement is true about deposition.
Deposition is a phase transition that occurs when a gas is converted directly to a solid without passing through the liquid state. During deposition, energy is released by the gas particles and absorbed by the surface, resulting in a decrease in the energy of the gas particles and an increase in the energy of the surface particles.As a result, the substance releases heat energy as it changes from a gas state to a solid state.
Therefore, the correct option is:Heat energy released by the substance.
Learn more about heat energy,here:
https://brainly.com/question/29210982
#SPJ5
A student made a sketch of a potential energy diagram to represent an exothermic reaction.
Explain, using complete sentences, why the diagram made by the student is correct or incorrect. Be sure to also explain what the values of X and Y represent. (10 points)

Answers
The profile shown is not for an exothermic reaction because the energy of the products is greater than that of the reactants.
What is an exothermic reaction?The term exothermic reaction refers to a type of reaction in which energy is given out during the reaction. In this type of reaction, the energy of the reactants is greater than the energy of the products. This is which it is possible for the excess energy to be given out in the reaction.
The potential energy diagram is used to show the profile of a reaction. We could look at the diagram and deduce the energy of the reactants and the products as well as the enthalpy change of the reaction which is the difference between the enthalpies of the reactants and the products.
If we have a good look at the potential energy diagram as we have in that image, the energy of the products is greater than that of the reactants hence the enthalpy change of the reaction is positive. Thus this is an endothermic reaction profile as shown.
Learn more about exothermic reaction:https://brainly.com/question/10373907
#SPJ1
If you had 4.8 x 10^23 molecules of butane, how many moles would this amount represent? (SHOW ALL WORK FOR BRAINLIEST)
Answers
Answer:
The mole is simply a very large number that is used by chemists as a unit of measurement.
Explanation:
The mole is simply a very large number,
6.022
×
10
23
, that has a special property. If I have
6.022
×
10
23
hydrogen atoms, I have a mass of 1 gram of hydrogen atoms . If I have
6.022
×
10
23
H
2
molecules, I have a mass of 2 gram of hydrogen molecules. If I have
6.022
×
10
23
C
atoms, I have (approximately!) 12 grams.
The mole is thus the link between the micro world of atoms and molecules, and the macro world of grams and litres, the which we can easily measure by mass or volume. The masses for a mole of each element are given on the periodic table as the atomic weight. So, if have 12 g of
C
, I know, fairly precisely, how many atoms of carbon I have. Given this quantity, I know how many molecules of
O
2
are required to react with the
C
, which I could measure by mass or by volume.
Use the following reaction to define redox reaction, half-reaction, oxidizing agent, reducing agent: 4Na(s) + O₂(g) → 2Na₂O(s)
Answers
Answer:
in this reaction na got oxidized and got reduced, therefore, in a redox reaction, the reactants get both oxidized and reduced
a chemical bond can be compared to a coiled spring. just as it takes energy to stretch a spring, energy is needed to stretch a chemical bond. a bond stretches and contracts as it vibrates. the frequency of molecular vibrations of organic molecules lies in the infrared region of the electromagnetic spectrum. infrared light activates these vibrations, a process that consumes energy. in ir spectroscopy, a sample of a molecule is irradiated with light in the ir portion of the electromagnetic spectrum. the energy of the infrared radiation after it has passed through the sample is then measured. a decrease in the energy of a particular wavelength indicates that the molecule has absorbed this energy by undergoing some type of vibration. the energy of the transmitted radiation is plotted as a function of the frequency of the infrared radiation. the plot appears as a series of peaks and is called an infrared spectrum.
Answers
In IR spectroscopy, a sample of a molecule is irradiated with light in the IR portion of the electromagnetic spectrum. The energy of the infrared radiation after it has passed through the sample is then measured.
A decrease in the energy of a particular wavelength indicates that the molecule has absorbed this energy by undergoing some type of vibration. This is because when a chemical bond stretches and contracts, it vibrates at a specific frequency, which corresponds to a specific wavelength in the IR region of the electromagnetic spectrum. When the sample absorbs IR light at a specific frequency, it causes the bond to vibrate, consuming energy. An IR spectrum is a plot of the energy of the transmitted radiation as a function of the frequency of the infrared radiation. The plot appears as a series of peaks, each of which corresponds to a specific bond vibration in the molecule. The position and intensity of these peaks can provide information about the types of bonds and functional groups present in the molecule.
learn more about electromagnetic spectrum here:
brainly.com/question/23727978
#SPJ4
The volume of a gas is 325 mL when the temperature is 57°C. If the temperature is reduced to 10°C without changing the pressure, what is the new volume of the gas? Combined gas law 2 P₁V₁ T₁ P₂V2 T2 = 278.7 mL
Answers
Answer:
The new volume of the gas is 278.7 mL.
Explanation:
To solve this problem, we can use the combined gas law:
P₁V₁/T₁ = P₂V₂/T₂
where P is the pressure, V is the volume, and T is the temperature.
We can start by plugging in the given values for the initial state of the gas:
P₁ is not given, so we can assume it remains constant.
V₁ = 325 mL
T₁ = 57°C + 273.15 = 330.15 K
Now we can solve for P₂V₂/T₂:
P₂V₂/T₂ = P₁V₁/T₁
We want to solve for V₂, so we can rearrange the equation:
V₂ = (P₁V₁/T₁) * T₂/P₂
We are given that the pressure remains constant, so P₁ = P₂.
Now we can plug in the remaining values:
V₂ = (P₁V₁/T₁) * T₂/P₂
V₂ = (P₁ * 325 mL / 330.15 K) * (10°C + 273.15) / P₁
V₂ = 278.7 mL
Therefore, the new volume of the gas is 278.7 mL.
Hope I helped you!
An atomic model shows 19 protons, 20 neutrons, and 19 electrons. What is the mass number of the atom?
Answers
Answer:
32
Explanation:
i just know bc i already answered this question... and i dont have an explination
Esters, amines, and amides have many uses in medicine. Investigate one of the following drugs further: aspirin, Benadryl, or Tylenol and give its scientific name. What kind of functional groups does it contain?
Answers
Esters, amines, and amides have many uses in medicine, and so do carboxylic acids, such as aspirin. Aspirin is the drug that will be investigated further. Its scientific name is acetylsalicylic acid.
What kind of functional groups does Aspirin contain?Acetylsalicylic acid contains two functional groups: a carboxylic acid group (-COOH) and an ester group (-COO-CH₃). The carboxylic acid group is responsible for the acidic properties of aspirin and allows it to form salts with bases. The ester group is formed from the reaction between the carboxylic acid group of salicylic acid and acetic anhydride. This esterification reaction makes aspirin more soluble in organic solvents and less irritating to the stomach than salicylic acid.
Aspirin is a widely used medication that has anti-inflammatory, analgesic, and antipyretic properties. It works by inhibiting the production of prostaglandins, which are responsible for pain, inflammation, and fever. Aspirin is commonly used to treat headaches, fever, arthritis, and other inflammatory conditions. It is also used as a blood thinner to prevent heart attacks and strokes.
To know more about aspirin, visit:
https://brainly.com/question/23878261
#SPJ1
In this question you should assume that all gases behave ideally.
Hydrogen and iodine react reversibly in the following reaction. The system reaches dynamic
equilibrium.
H2(g) + I2(g) 2HI(g) ∆H=–9.5kJmol–1 Which statement must be true for the Kp of this equilibrium to be constant?
A The partial pressures of H2, I2 and HI are equal.
B The external pressure is constant.
C The forward and reverse reactions have stopped.
D The temperature is constant.
Answers
Answer:
B
Explanation:
The number of moles of each reactant is equal, there by pressure has no effect on the chemical system. Pressure is constant
12 Igneous rocks are formed by (1) sedimentation (2) fossilization (3) volcanic activity (4) freezing/thawing cycles
Answers
According to the research, the correct option is 3, Igneous rocks are those formed from a molten product from volcanic activity.
What are Igneous rocks?They are rocks of high resistance, isotropy, brittleness and density that are created from the cooling and solidification of the magma, whether fragmented or compact, that form at erupting volcanoes, that is, this substance, made up of molten rocks and other elements, is found inside the planet.
When the cooling process takes place superficially and quickly, extrusive igneous rocks are produced, among which are obsidian and basalt, they usually appear after the eruption of a volcano, since the ejected lava solidifies.
Therefore, we can conclude that according to the research, the correct option is 3, Igneous rocks are those formed from a molten product from volcanic activity.
Learn more about Igneous rocks here: brainly.com/question/12990631
#SPJ1
b. How many kJ of heat are needed to completely vaporize 50.0g of water at 100°C? [Ans:113. kJ]
Answers
The amount, in kJ, of heat needed to completely vaporize 50.0g of water at 100°C is 118.8 kJ.
Heat of vaporization of waterThe heat needed to completely vaporize 50.0g of water at 100°C can be calculated using the following formula:
q = m x Hv
where:
q is the heat needed in joules (J)m is the mass of water in grams (g)Hv is the heat of vaporization of water which is approximately 40.65 kJ/mol at standard temperature and pressure.First, we need to convert 50.0g to moles by dividing by the molar mass of water which is approximately 18.015 g/mol3:
moles of water = 50.0 g / 18.015 g/mol moles of water = 2.776 mol
Thus:
q = (2.776 mol) x (40.65 kJ/mol) q = 112.8 kJ
In other words, 112.8 kJ of heat is needed to completely vaporize 50.0g of water at 100°C.
More on heat of vaporization can be found here: https://brainly.com/question/12625048
#SPJ1
Name the following compounds NH4CI
Answers
The compound NH4Cl or ammonium chloride is composed of two ions: ammonium ion (NH4+) and chloride ion (Cl-). The ammonium ion is a polyatomic cation made up of one nitrogen atom and four hydrogen atoms, while the chloride ion is a monatomic anion made up of one chlorine atom.
Would you expect deuterium,the hydrogen isotope with a mass of 2 amu, to emit light at the same wave lengths as hydrogen? why or why not? justify your answer using the Bohr model.
Answers
Deuterium (D, or 2H), also known as heavy hydrogen, is an isotope of hydrogen that has a nucleus made up of one proton and one neutron and has a mass twice that of regular hydrogen's nucleus (one proton). The atomic weight of deuterium is 2.014.
What are isotopes ?Isotopes are atoms with the same number of protons but different numbers of neutrons. They differ in mass, which affects their physical characteristics even though they have nearly identical chemical properties.
The most prevalent element in the universe, hydrogen, has isotopes called deuterium and tritium. All hydrogen isotopes have one proton, but tritium and deuterium also have one neutron each, making their ion masses heavier than those of protium, the only hydrogen isotope without a neutron.
Thus, deuterium,the hydrogen isotope with a mass of 2 amu, to emit light at the same wave lengths as hydrogen.
To learn more about the isotope, follow the link;
https://brainly.com/question/11680817
#SPJ1
If the oh- of a solution is 2.7x10-4m the poh of the solution is
Answers
Answer:
i dont knoe but yea just on here
Explanation:n:
hi hi hi hi
what is the molarity of a solution tgat contain 0.050 mol c12h22o11 in 0.215 g water
Answers
232.56M
Explanations:The formula for calculating the molarity of the solution is expressed as:
\(molarity=\frac{moles\text{ of solute}}{volume\text{ of solution}}\)Given the following parameters
moles of solute = 0.05moles
mass of solution (water) = 0.215g
Determine the volume of the solution
\(\begin{gathered} volume=\frac{mass}{density} \\ volume\text{ of water}=\frac{0.215g}{1000gL^{-1}} \\ volume\text{ of water}=0.000215L \end{gathered}\)Determine the molarity of the solution
\(\begin{gathered} molarity\text{ of the solution}=\frac{0.05}{0.000215} \\ molarity\text{ of the solution}=232.56M \end{gathered}\)Hence the molarity of a solution that contain 0.050 mol C12H22O11 in 0.215 g water is 232.56M
HELP PLEASEUse the molar volume of a gas at SATP to determine the
following values at SATP:
(a) the amount of nitrogen in 44.8 L of pure gas
(b) the volume (in litres) of 4.8 mol of propane gas, C3H,(g)
(c) the mass of carbon dioxide in 34.6 L of carbon dioxide
gas, CO₂(g)
(d) the volume (in mL) of 1250 g of methane, CH₂(g)
(e) the amount of oxygen in 36.5 L of 02 gas
Answers
The most common example is the molar volume of a gas at standard temperature and pressure (STP), which is equal to 22.4 L for 1 mole of any ideal gas at 273.15 K and 1 atm of pressure.
What are the applications of the Ideal Gas Law- Molar Volume?Stoichiometry refers to the quantitative relationship between reactants and products. Calculating the quantities of reactants needed to make a given quantity of products, or the quantities of products resulting from a given quantity of reactants, is required in stoichiometric problems. Gas laws must be taken into account for the calculation if one or more reactants or products in a chemical reaction are gases. The findings of ideal gas law applications are often accurate to within 5%. We go over a few key ideas that are crucial for solving Stoichiometry Problems involving Gases in the sections below.
Stoichiometry and gas laws both rely on the mole notion as their foundation. A mole is an exact measurement of a substance. Based on the quantity of identities, a mole is a unit (i.e. atoms, molecules, ions, or particles). The number of identities in a mole of anything is equal to the number of atoms in exactly 12 grams of carbon-12, the most common isotope of carbon.
Learn more about Ideal Gas Law- Molar Volume: https://brainly.com/question/15132707
#SPJ1