2) A student was working on an investigation to measure the relative activity
of an enzyme at various pH values. He collected the following data:
pH 2, enzyme activity 10; pH 8, enzyme activity 50;
pH 12, enzyme activity 10; pH 4, enzyme activity 20;
pH 6, enzyme activity 40; pH 10, enzyme activity 40
Answers
Answer:
THE LAST ONE
Explanation:
Related Questions
A solution is prepared by dissolving a solid in a liquid. What does the vapor pressure of the liquid depend on?A.the solution molarityB.the solution molalityC.the mole fraction of the liquidD.the mass percent of the solid
Answers
• The factors that influence vapour pressure is amongst others surface area.
• So the most suitale nswer will be the mole raction of the liquid will influence vapour pressure.
• Option C is the most closest answer,.
The total number of nearest neighbor atoms surrounding a given atom in a closest packed lattice is ___
A. 2
B. 4 C. 6
D. 8
E. 12 F, 16
Answers
why would Alcohol be effective in cleaning a dirty penny
Answers
Answer:
The alcohol cleans the penny due to the acid in the vinegar. A chemical reaction between the alcohol removes copper oxide. Copper oxide is what causes pennies to become dull. Salt is also a key ingredient in the other cleaning agents, thus making them effective in cleaning the pennies.
Which of the following is a complete description of the velocity of an object in motion?
Answers
Enough of a monoprotic acid is dissolved in water to produce a 1.78 M solution. The pH of the resulting solution is 2.71 . Calculate the Ka for the acid.
Answers
To calculate the Ka for the monoprotic acid, we can use the relationship between the concentration of the acid, the concentration of the conjugate base, and the pH of the solution. The Ka value for the monoprotic acid is approximately 5.7 × 10⁻⁵.
In a monoprotic acid, the acid (HA) donates one proton (H+) to water, forming the conjugate base (A-).
The dissociation of the acid can be represented as follows:
HA(aq) ⇌ H+(aq) + A-(aq)
Given that the pH of the solution is 2.71, we know that the concentration of H+ is 10^(-pH), which is 10⁻²°⁷¹ in this case.
Let's assume that the initial concentration of the acid HA is represented by [HA]_0.
At equilibrium, the concentration of H+ is equal to the concentration of A-. Therefore, [H+] = [A-].
Using the given concentration of the acid solution (1.78 M), we can assume that the concentration of the acid HA at equilibrium is (1.78 - [H+]) M.
The equilibrium expression for the acid dissociation is given by the equation:
Ka = ([H+][A-])/[HA]
Substituting the values we have:
Ka = ([H+][H+])/[(1.78 - [H+])]
Now, we can substitute [H+] = 10⁻²°⁷¹ into the equation and solve for Ka:
Ka = (10⁻²°⁷¹ * 10⁻²°⁷¹)/[(1.78 - 10⁻²°⁷¹)]
Ka ≈ 5.7 × 10⁻⁵
Therefore, the Ka value for the monoprotic acid is approximately 5.7 × 10⁻⁵.
For more details regarding monoprotic acid, visit:
https://brainly.com/question/31116483
#SPJ4
how would you know if your sample contained impurities when taking the melting point?
Answers
The presence of impurities in a sample can be detected by observing changes in the melting point of the substance.
When an impure sample is heated, its melting point range becomes broader and its melting point decreases. This is because the impurities disrupt the crystal lattice structure of the substance, making it easier to break apart and melt. In contrast, a pure substance will melt at a sharp and well-defined melting point.
Therefore, if the melting point range of a sample is broad and has a lower melting point than the expected value of the pure substance, it may contain impurities. Additionally, the appearance of a plateau or depression on the melting point curve is also indicative of impurities.
To confirm the presence of impurities, one can perform a mixed melting point test. This involves mixing a small amount of the sample with a known pure compound and taking the melting point of the mixture. If the melting point of the mixture is depressed and has a lower range than that of the pure compound alone, then the sample contains impurities. If the melting point range remains the same and is sharp, then the sample is likely pure.
To learn more about Melting Point click here
https://brainly.com/question/5753603
#SPJ11
Fires are classified according to their properties, which relate to the nature of the fuel. What class of fire has a metal fuel
Answers
Fires are classified according to their properties, fires fueled by metals fall under Class D fire category.
Fires are classified into different classes based on the nature of the fuel. One of the classes is Class D fire, which involves fires fueled by metals. Metals such as magnesium, sodium, potassium, and titanium can ignite and burn under certain conditions. Class D fires require specific extinguishing agents, such as dry powder extinguishers, to effectively control and suppress them.
To know more about metal visit:
https://brainly.com/question/29404080
#SPJ11
gnoring electron repulsion, the ground state energy of helium is related to that of hydrogen by a factor:
Answers
Ignoring electron repulsion, the ground state energy of helium can be related to that of hydrogen by a factor of 4.
This is due to the fact that the ground state energy of an atom is determined by the total energy of its electrons, which is primarily determined by their positions and motions around the nucleus. In the case of hydrogen, the ground state energy is determined by the electron's interaction with the positively charged nucleus. This interaction results in a specific energy level that the electron can occupy.
However, in the case of helium, there are two electrons that occupy the same space and interact with the same nucleus. This results in the phenomenon known as electron repulsion, which makes the energy level of helium higher than that of hydrogen. To ignore electron repulsion means to assume that the two electrons in helium do not interact with each other, which allows us to treat helium as if it had only one electron.
By doing this, the energy level of helium becomes comparable to that of hydrogen, and we can relate them by a factor of 4, which is the ratio of the charge of the helium nucleus to that of the hydrogen nucleus. In summary, ignoring electron repulsion allows us to relate the ground state energy of helium to that of hydrogen by a factor of 4, which is a useful approximation in certain situations.
know more about electron repulsion here:
https://brainly.com/question/10271048
#SPJ11
4. The only two elements in alkanes, alkenes and alkynes are...
(1) carbon and nitrogen
(2) carbon and hydrogen
(3) oxygen and nitrogen
(4) oxygen and hydrogen
Answers
Hope that helps!
why there is presence of double or triple bond between carbon?
Answers
Answer:
Alkynes
Explanation:
Alkynes are hydrocarbons which contain carbon-carbon triple bonds. Their general formula is CnH2n-2 for molecules with one triple bond (and no rings). Alkynes undergo many of the same reactions as alkenes, but can react twice because of the presence of the two p-bonds in the triple bond.
What measuring tool is used to find the volume of a shoe box?
A.water displacement
B.scale
C.beaker
D.ruler
Answers
Answer:
D. Ruler
Explanation:
Use the formula to find the volume of a cuboid by measuring the length, width, and height and multiplying all of them by each other.
suppose the reaction is carried out starting with 129 g of ca3(po4)2 and 97.4 g of h2so4. which substance is the limiting reactant?
Answers
The limiting reactant is Ca₃(PO₄)₂.
To determine the limiting reactant in a chemical reaction, we need to compare the amount of moles of each reactant and see which one is completely consumed first.
The balanced chemical equation for the reaction between calcium phosphate (Ca3(PO4)2) and sulfuric acid (H₂SO₄) is:
3Ca₃(PO₄)₂ + 2H₂SO₄ → 6CaSO₄ + H₄P₂O₇
First, let's convert the given masses of each reactant to moles:
moles of Ca₃(PO₄)₂ = 129 g / (3 x 310.18 g/mol) = 0.139 mol
moles of H₂SO₄ = 97.4 g / (2 x 98.08 g/mol) = 0.993 mol
According to the balanced chemical equation, it takes 3 moles of Ca₃(PO₄)₂ and 2 moles of H₂SO₄ to produce the products. So, we need to multiply the amount of moles of each reactant by the appropriate stoichiometric coefficient in the balanced equation to see which reactant is completely consumed first:
For Ca₃(PO₄)₂: 0.139 mol x (2/3) = 0.093 mol of H₂SO₄ required
For H₂SO₄: 0.993 mol x (3/2) = 1.49 mol of Ca₃(PO₄)₂ required
From the above calculations, we can see that 0.139 mol of Ca₃(PO₄)₂ require 0.093 mol of H₂SO₄ for complete reaction. But we have 0.993 mol of H₂SO₄ available which is much greater than the required amount of H₂SO₄. Therefore, the limiting reactant is Ca₃(PO₄)₂.
To know more about limiting reactant here
https://brainly.com/question/2948214
#SPJ4
sodium and oxygen react to produce Sodium Oxide. How many moles of oxygen are needed to produce 11.5 grams of sodium oxide
Answers
INFORMATION:
We know that:
- sodium and oxygen react to produce Sodium Oxide
-
A student carefully placed 18.1 g of sodium in a reactor supplied with an excess quantity of chlorine gas. When the reaction was complete, the student obtained 39.6 g of sodium chloride. How many grams of chlorine gas reacted?
Answers
Answer:
The mass of chlorine gas that reacted ≅ 24.03 g
Explanation:
From the given information:
The number of moles of NaCl formed = mass of NaCl/molar mass
mass of NaCl = 39.6 g
molar mass = (23 + 35.5) g/mol = 58.5 g/mol
∴
The number of moles of NaCl formed = 39.6 g / 58.5 g/mol
The number of moles of NaCl formed = 0.6769 g/mol
Thus, the mass of chlorine gad that reacted = 0.6769 × 35.5
The mass of chlorine gas that reacted = 24.02995 g
The mass of chlorine gas that reacted ≅ 24.03 g
Does indium react with acid?
Answers
In the following compound (NaCl), can you please tell me the following:
Is it an ionic or covalent compound?
How many valence electrons does Na have?
How many valence electrons does Chlorine have?
How many electrons are gained and lost by each atom
Answers
Answer:
Ionic
Na has one valence electron
Chlorine has seven
Sodium loses one electron
Chlorine gains one electron
the reaction a 2b → c is found to be first order with respect to both a and b. when the concentration of a is 0.200 m and the concentration of b is 0.400 m, the reaction rate is 0.00700 m/s. what is the numerical value of k
Answers
Option E) In the reaction A+ 2B-C that is found to be first order with respect to both a (0.200 m concentration and b (0.400 m concentration) the numeric value of k is 0.0875
What is Concentration?Concentration in chemistry terms is calculated by dividing a constituent's abundance by the mixture's present total volume.
Mass concentration, molar concentration, number concentration, and volume concentration are four different categories that can be described in a mathematical description.
A + 2B → C
Rate = K [A] [B]
Here,
[A] = 0.2 M [B]
⇒ [A] = 0.4 M
rate = 0.007 M/s
Therefore, rate = K [A] [B]
⇒ 0.007 = K (0.2) (0.4)
⇒ K = 0.0875 M^(-1) S^(-1)
Thus, option E is correct.
To know more about concentration, visit:
https://brainly.com/question/13872928
#SPJ4
Complete question
The reaction A+ 2B-C is found to be first order with respect to both A and B. When the concentration of A is 0.200 M and the concentration of B is 0.400 M, the reaction rate is 0.00700 M/s. What is the numerical value of k for this reaction (with appropriate units where time is in seconds and concentration is in M, as needed).
A) 0.0438
B) 0.0140
C) 11.4
D) 5.60 x 10^(-4)
E) 0.0875
How much water is needed to make 7.2moles of glucose?\(6CO2 + 6H2O -\ \textgreater \ C6H12O6 + 6O2\)
Answers
Approximately 777.6 grams of water is needed to make 7.2 moles of glucose based on the balanced equation.
The balanced equation provided is:
6CO2 + 6H2O -> C6H12O6 + 6O2
From the equation, we can see that for every 6 moles of water (H2O), 1 mole of glucose (C6H12O6) is produced. Therefore, we need to determine the amount of water required to produce 7.2 moles of glucose.
The mole ratio between water and glucose is 6:1. This means that for every 6 moles of water, we obtain 1 mole of glucose. To find the amount of water needed for 7.2 moles of glucose, we set up a proportion using the mole ratio:
(6 moles H2O / 1 mole glucose) = (x moles H2O / 7.2 moles glucose)
Solving for x, we can cross-multiply:
6 moles H2O * 7.2 moles glucose = x moles H2O * 1 mole glucose
43.2 moles H2O = x moles H2O
Therefore, we need 43.2 moles of water to produce 7.2 moles of glucose.
To convert moles of water to grams, we need to know the molar mass of water, which is approximately 18 g/mol. Using the molar mass, we can calculate the mass of water needed:
Mass of water = moles of water * molar mass of water
Mass of water = 43.2 moles * 18 g/mol
Mass of water = 777.6 g
Therefore, approximately 777.6 grams of water is needed to make 7.2 moles of glucose based on the balanced equation.
for more such question on glucose visit
https://brainly.com/question/397060
#SPJ8
why does the d block start in the fourth row of the periodic table
Answers
Answer:
Why does D-block start on the fourth row of the periodic table?
Explanation:
The require more energy to reach 3d than 4s, and they fill up 4p before 4d, and so forth. ... The energy is lower for 6s than 4f, and 4f and 5f are lower in energy than the final D level.
please i just want the answer for the boxes if you know the right answer please tell me please
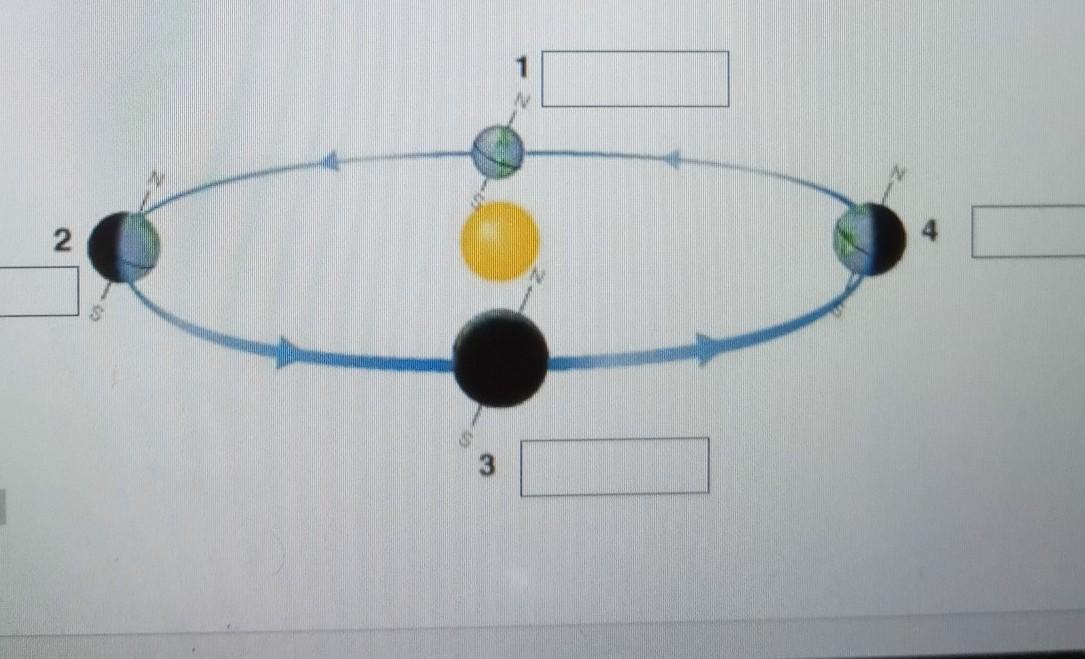
Answers
Answer:
1. summer 2 spring 3 winter 4 fall/autumn
determine the excess reactant and calculate the mass of the remaining excess reactant after 35.0 grams of fe2o3 and 30.0 grams of al react.
Answers
The excess reactant is Al, and the mass of the remaining excess Al after the reaction is 16.5 grams.
To determine the excess reactant and the mass of the remaining excess reactant, we need to first write and balance the chemical equation for the reaction between \(Fe_{2} O _{3}\) and Al:
2 \(Fe_{2} O _{3}\) + 2 Al → \(Al_{2} O_{3}\) + 4 Fe
From the balanced equation, we can see that the stoichiometric ratio of \(Fe_{2} O _{3}\)to Al is 2:2, or 1:1. This means that both reactants are consumed in equal amounts, and neither is in excess.
To confirm this, we can calculate the amount of product formed by each reactant and compare it to the actual amount of product formed:
For \(Fe_{2} O _{3}\) : Using its molar mass of 159.69 g/mol, the number of moles of Fe2O3 is 35.0 g / 159.69 g/mol = 0.2192 mol. According to the balanced equation, 2 moles of \(Fe_{2} O _{3}\) produces 4 moles of Fe. Therefore, the number of moles of Fe produced from 0.2192 mol of \(Fe2O _{3}\) is (4/2) x 0.2192 = 0.4384 mol.
For Al: Using its molar mass of 26.98 g/mol, the number of moles of Al is 30.0 g / 26.98 g/mol = 1.111 mol. According to the balanced equation, 2 moles of Al produces 4 moles of Fe. Therefore, the number of moles of Fe produced from 1.111 mol of Al is (4/2) x 1.111 = 2.222 mol.
We can see that Al produces twice as much Fe as \(Fe_{2} O _{3}\), indicating that \(Fe_{2} O _{3}\) is the limiting reactant and Al is in excess. However, we also need to calculate the mass of the remaining excess Al:
The number of moles of Al that reacted is 1.111 - 0.5 (since 2 moles of Al react with 2 moles of \(Fe_{2} O _{3}\)) = 0.611 mol.
The mass of the remaining excess Al is therefore 0.611 mol x 26.98 g/mol = 16.5 g (rounded to one decimal place).
Therefore, the excess reactant is Al, and the mass of the remaining excess Al after the reaction is 16.5 grams.
Learn more about chemical equation
https://brainly.com/question/30087623
#SPJ4
How many atoms are in 76.15 grams of fe
urgently needed, will mark brainliest
Answers
Answer:
See below
Explanation:
From periodic table
mole weight of Fe is 55.845 gm / mole
76.15 gm / 55.845 gm/mol * 6.022 x 10^23 atoms/mole =
8.212 x 10^23 atoms ( four signif. digits)
Elements in a groups have the same number of ___ and the same ____.
Answers
Answer:
Protons, electrons
Explanation:
Elements in a groups have the same number of protons and the same electrons.
Calculate the equilibrium concentrations of reactant and products when 0.363 moles of cocl2(g) are introduced into a 1.00 l vessel at 600 k.
Answers
The equilibrium concentrations of the reactant (CoCl2(g)) and products (Co(g) and Cl2(g)) when 0.363 moles of CoCl2(g) are introduced into a 1.00 L vessel at 600 K can be expressed as [CoCl2(g)] = (0.363 - x) moles/L, [Co(g)] = x moles/L, and [Cl2(g)] = x moles/L
To calculate the equilibrium concentrations of reactant and products, we need to use the equilibrium constant (K) expression and the stoichiometry of the balanced chemical equation.
First, let's write the balanced chemical equation for the reaction:
CoCl2(g) ⇌ Co(g) + Cl2(g)
Next, we need the value of the equilibrium constant (K) at 600 K. Unfortunately, the equilibrium constant value is not provided in the question. Without the equilibrium constant, we cannot determine the exact equilibrium concentrations of the reactant and products.
However, we can still calculate the equilibrium concentrations using the ICE (Initial, Change, Equilibrium) table method. We start by writing down the initial concentrations of the reactant and products, which is 0.363 moles of CoCl2(g) in a 1.00 L vessel.
Next, we assume x moles of Co(g) and Cl2(g) are formed or consumed at equilibrium. Using the stoichiometry of the balanced equation, we know that the change in concentration of Co(g) and Cl2(g) is x moles.
Therefore, the equilibrium concentrations are as follows:
[CoCl2(g)] = (0.363 - x) moles/L
[Co(g)] = x moles/L
[Cl2(g)] = x moles/L
Without the value of the equilibrium constant, we cannot calculate the exact equilibrium concentrations. However, we can express the concentrations in terms of x, which represents the change in moles at equilibrium.
In summary, the equilibrium concentrations of the reactant (CoCl2(g)) and products (Co(g) and Cl2(g)) when 0.363 moles of CoCl2(g) are introduced into a 1.00 L vessel at 600 K can be expressed as [CoCl2(g)] = (0.363 - x) moles/L, [Co(g)] = x moles/L, and [Cl2(g)] = x moles/L.
Learn more about equilibrium concentrations from given link: https://brainly.com/question/13414142
#SPJ11
74.5 g of was dissolved in 1000. of water. What is the molality of the solution? (Molar mass of KCl = 74.5g / m * o * l ; Density of water = 1.00 g/mol )
Answers
Answer: 1.00 m
Explanation:
What term is used by chemists to quantitatively describe a solution in which a relatively small amount of solute is dissolved?
Answers
Which type of basin forms at transform boundaries?
rift
wedge
arc
strike-slip
Answers
In an ecosystem, strike-slip type of basin are formed at transform boundaries.
What is an ecosystem?Ecosystem is defined as a system which consists of all living organisms and the physical components with which the living beings interact. The abiotic and biotic components are linked to each other through nutrient cycles and flow of energy.
Energy enters the system through the process of photosynthesis .Animals play an important role in transfer of energy as they feed on each other.As a result of this transfer of matter and energy takes place through the system .Living organisms also influence the quantity of biomass present.By decomposition of dead plants and animals by microbes nutrients are released back in to the soil.
Learn more about ecosystem,here:
https://brainly.com/question/13979184
#SPJ7
Which one of these statements is usually true about waves?
A.All kinds of waves are the same size.
B.All waves move at the same speed.
C.Waves don't move matter, just energy.
D.Some waves do not move in a pattern.
Answers
Answer: C. Waves don't move matter, just energy.
Explanation:
The ideal gas law is equivalent to Charles's law when
a) volume equals 22.4 L
b)R equals 0
c)number of moles and temperature are constant
d)number of moles and pressure are constant
Answers
The ideal gas law is equivalent to Charles's law when number of moles and pressure are constant (option D)
Ideal gas equationPV = nRT
Where
P is the pressure V is the volume n is the number of mole R is the gas constant T is the temperature Charles' lawCharles' law states as follow:
V₁ / T₁ = V₂ / T₂
Where
V₁ is the initial volume T₁ is the initial temperature V₂ is the new volume T₂ is the new temperature How to relate ideal gas equation to Charles' lawPV = nRT
Divide both side by T
PV / T = nR
Divide both side by P
V / T = nR / P
Let nR / P be costant
V / T = constant
Thus,
V₁ / T₁ = V₂ / T₂
From the above illustrations, we can conclude that the ideal gas equation will become Charles' law equation if the number of mole and pressure are constant (Option D)
Learn more about ideal gas equation:
https://brainly.com/question/4147359
#SPJ1
NEED HELP AS SOON AS POSSIBLE please


Answers
0.08 gram mass of magnesium oxide will be formed.
What is mass?Mass is an intrinsic property in the mass of a body. It was the traditionally believed to the be related to the quantity of matters in the physical body, until it is discovery of the atom and particle in physics.
Mass is quantitative measure of inertia a fundamental quantity of all matters.
2Mg + O 2→2MgO
Molar mass of Mg =24 g
Molar mass of MgO =40 g
No. of moles of O2=6.022×10^20/6.022×10^23
=0.001 mole of O2
No. of moles of Mg=0.486/24
=0.02 moles of Mg
As moles of O 2
are less than 0.01, O 2
is limiting reagent and it is 0.002 moles of MgO is formed.
⇒0.002×40=0.08 g of MgO
To know more about mass click-
https://brainly.com/question/19385703
#SPJ1