Answers
Related Questions
ACTIVITY: SOLUTION CONCENTRATION VS. CONDUCTIVITY
Here is your goal for this lesson:
Graph experimental data and interpret results for peer review
A chemistry student carried out an experiment with a conducting apparatus (ammeter) similar to the one below. This ammeter measures in milliamperes (mA). The following data was taken.
Solution Reading
0.1 M H2SO4 150 mA
0.1 M Ba(OH)2 150 mA
To 30 mL of the Ba(OH)2 solution, 10 mL portions of H2SO4 were added until a total of 50 mL of H2SO4 were used. The following results were recorded.
DATA TABLE
Total H2SO4 Added Meter Reading Observations
0 mL 150 mA Ba(OH)2 and H2SO4 clear, colorless
10 mL 65 mA milky white precipitate forms
20 mL 31 mA more precipitate forms
30 mL 0 mA precipitate heavy and settles
40 mL 29 mA no added precipitate seen to form
50 mL 62 mA no change seen
Questions
1. Did you plot the data?
yes
no
2. Did you label your graph axes?
no
yes
3. Did you give your graph a title?
no
yes
4. Does the Ba(OH)2 solution contain ions?
yes
no
5. Does the H2SO4 solution contain ions?
yes
no
6. Explain the data.
Is there any evidence that a reaction has occurred?
7. Does the conductivity increase or decrease?
8. Does the number of ions in solution increase, decrease, or remain constant?
9. What is the indicator of the number of ions in solution?
10. How does this evidence indicate that the reaction has occurred between ions?
11. The Ba(OH)2 dissociates as Ba+2 + 2 OH-. H2SO4 dissociates as 2 H+ + SO4-2.
Write a balanced equation for this reaction.
12. When the conductivity is at a minimum, what must be true about the amount of Ba(OH)2?
13. Why does it not conduct at this low point?
14. Why does it conduct more before and after this minimum point?

Answers
Answer:
the amount of Ba(OH)2 compared to H2SO4?
The Ba(OH)2 dissociates as Ba+2 + 2 OH-. H2SO4 dissociates as 2 H+ + SO4-2.
Why does it not conduct at this low point?
Why does it conduct more before and after this minimum point
Can someone please help with this I am not that great at chemistry. Thankyou fo any help that I ge
PLASE ANSWER NOW! How many days and hours does a lunar cycle last? A lunar cycle last ___ days and ___ hours.
Answers
Answer:
How Long Is The Lunar Cycle: Each lunar cycle lasts 27 days, 7 hours and 43 minutes. This means that the moon takes almost 28 days to complete a full orbit around the earth. Over this course, the moon goes through several phases, which alters its appearance and even the pattern of movement in the sky.
Explanation:
hope this helps plz mark brainliest
Answer:
27 days and 7 hours
Explanation:
im guessing on this one but im pretty sure its correct it also says that our moon completes the cycle in 29.5 days.
The Law of Conservation of Mass states that mass changes before and after a change.
True
False
Answers
Describe the environment of Antarctica.
Answers
Answer:
Antarctica is a desert. It does not rain or snow a lot there. When it snows, the snow does not melt and builds up over many years to make large, thick sheets of ice, called ice sheets.
Explanation:
Balance the following reaction. A coefficient of "1" is understood. Choose option "blank" for the correct answer if the
coefficient is "1".
C₂H6+
02-
CO₂ +
✓ H₂O
Answers
Answer:
C₂H6 + O2 → 2CO2 + 3H2O
A chemist determines that a substance is composed of 30.4% nitrogen by mass and 69.6% oxygen by mass. The molar mass of the compound is 230.5 g/mol.
Answers
A chemist determines that a substance is composed of 30.4% nitrogen by mass and 69.6% oxygen by mass. The molar mass of the compound is 230.5 g/mol, the molecular formula is NO₂.
We must compute the empirical formula in order to ascertain the compound's chemical composition.
If we have 100 grams of the compound.
This suggests we have:
30.4 g of nitrogen
69.6 g of oxygen
Now, we have to convert the mass of each element to moles.
The molar mass of nitrogen (N) = 14.01 g/mol
the molar mass of oxygen (O) = 16.00 g/mol.
Number of moles of nitrogen (N):
2.17 mol
Number of moles of oxygen (O):
4.35 mol
The simplest whole-number ratio between the moles of nitrogen and oxygen must now be determined. To calculate the ratio, we divide both numbers by the smaller value.
Moles N / moles O = 2.17 mol / 2.17 mol = 1.00
Moles O / moles O = 4.35 mol / 2.17 mol = 2.00
The ratio is approximately N₁O₂.
We divide the subscripts by their greatest common divisor to obtain the simplest ratio, since we are looking for the empirical formula. The empirical formula is NO₂ since the greatest common divisor in this situation is 1.
The molecular formula of the compound is NO₂.
Learn more about molecular formula, here:
https://brainly.com/question/11203434
#SPJ1
can u pls help me with this question

Answers
What type of reaction is this?

Answers
Cu + O2 ---> CuO2 -The first reaction is a combustion reaction
2 HCl + Mg → H2 + MgCl2- The second reaction is a Single replacement reaction
What is a combustion reaction?A combustion reaction is a type of chemical reaction that occurs between a fuel and an oxidizer in the presence of heat or light, resulting in the release of energy in the form of heat and light.
In other words, it is a reaction in which a substance reacts with oxygen to produce heat and light.
Combustion reactions are important in many aspects of daily life, including the burning of fossil fuels for energy production, the combustion of wood or other materials for heating or cooking, and the combustion of fuels in internal combustion engines.
Learn more about combustion reaction:https://brainly.com/question/30562669
#SPJ1
Part 1(Picture 1):
Peptides isolated from rapeseed that may lower blood pressure have the following sequence of amino acids.
Part A
At physiological pH the N-terminus of an amino acid exists as the ammonium ion, and the C-terminus exists as the carboxylation ion.
Draw the structure of Arg-Ile-Tyr.
Draw the molecule on the canvas by choosing buttons from the Tools (for bonds and charges), Atoms, and Templates toolbars, including charges where needed.
Part 2(Picture 2):
Part A
What are the amino acids in the peptide?
Spell out the full names of the compounds. Enter your answers separated by a comma.
Part B
How would you name the dipeptide in the peptide?
Spell out the full name of the compound.


Answers
The structure of Arg-Ile-Tyr is given below:
What are Peptides?Peptides make up short chains of amino acids, which act as the fundamental units for building proteins. Within these chains, peptides usually consist of no more than 50 amino acids in contrast to proteins that are made up of significantly longer structures.
Found throughout various sources such as plants, animals, and bacteria, peptides play crucial roles in multiple biological processes including signaling, enzyme activity, and immune response.
Additionally, artificially synthesized peptides serve several purposes within the medicine, cosmetics, and food industries. Scientists are also exploring certain peptide's potential therapeutic advantages mainly pertaining to anti-inflammatory, antimicrobial, and anticancer activities.
Read more about peptides here:
https://brainly.com/question/21884818
#SPJ1


Acetylsalicylic acid, C9H8O4, is the pain reliever in many
headache medications. A typical tablet contains 250.0 mg
of acetylsalicylic acid.
(a) Calculate the amount of acetylsalicylic acid in the tablet.
(b) Calculate the number of hydrogen atoms in the amount
of acetylsalicylic acid in the tablet determined in (a).
Answers
The amount of acetylsalicylic acid in the tablet is 0.001389 moles and the number of hydrogen atoms in the amount of acetylsalicylic acid in the tablet is 6.69 × 10²¹.
(a)
The formula of acetylsalicylic acid is C₉H₈O₄.
Molar mass of C₉H₈O₄ = 9 × atomic mass of C + 8 × atomic mass of H + 4 × atomic mass of O
= 9 × 12 + 8 × 1 + 4 × 16
= 108 + 8 + 64
= 180
No of moles of acetylsalicylic acid = Given mass / molar mass
= 0.25 / 180
= 0.001389
Hence, the number of moles of acetylsalicylic acid is 0.001389 moles.
(b)
Number of hydrogen atoms present in 1 mole of acetylsalicylic acid = 6.023 × 10²³ atoms
Number of hydrogen atom present in 0.001389 moles of acetylsalicylic acid = 0.001389 × 6.023 × 10²³ = 0.008365 = 8.36 × 10²⁰ atoms.
Now there are 8 hydrogen atoms in acetylsalicylic acid so,
Number of atoms of hydrogen in acetylsalicylic acid = 8 × 8.36 × 10²⁰ atoms = 6.69 × 10²¹ atoms.
Hence, the number of atoms of hydrogen in 0.001389 moles of acetylsalicylic acid is 6.69 × 10²¹ atoms.
Learn more about number of atoms from the link given below.
https://brainly.com/question/496549
#SPJ1
Solve this organic transformation....use - Br2,CCl4,KOH,CH3OH,Hg+2,diluted H2SO4, PCC,HBr,Mg,Dry ether,Na,H2,Pd,quinoline
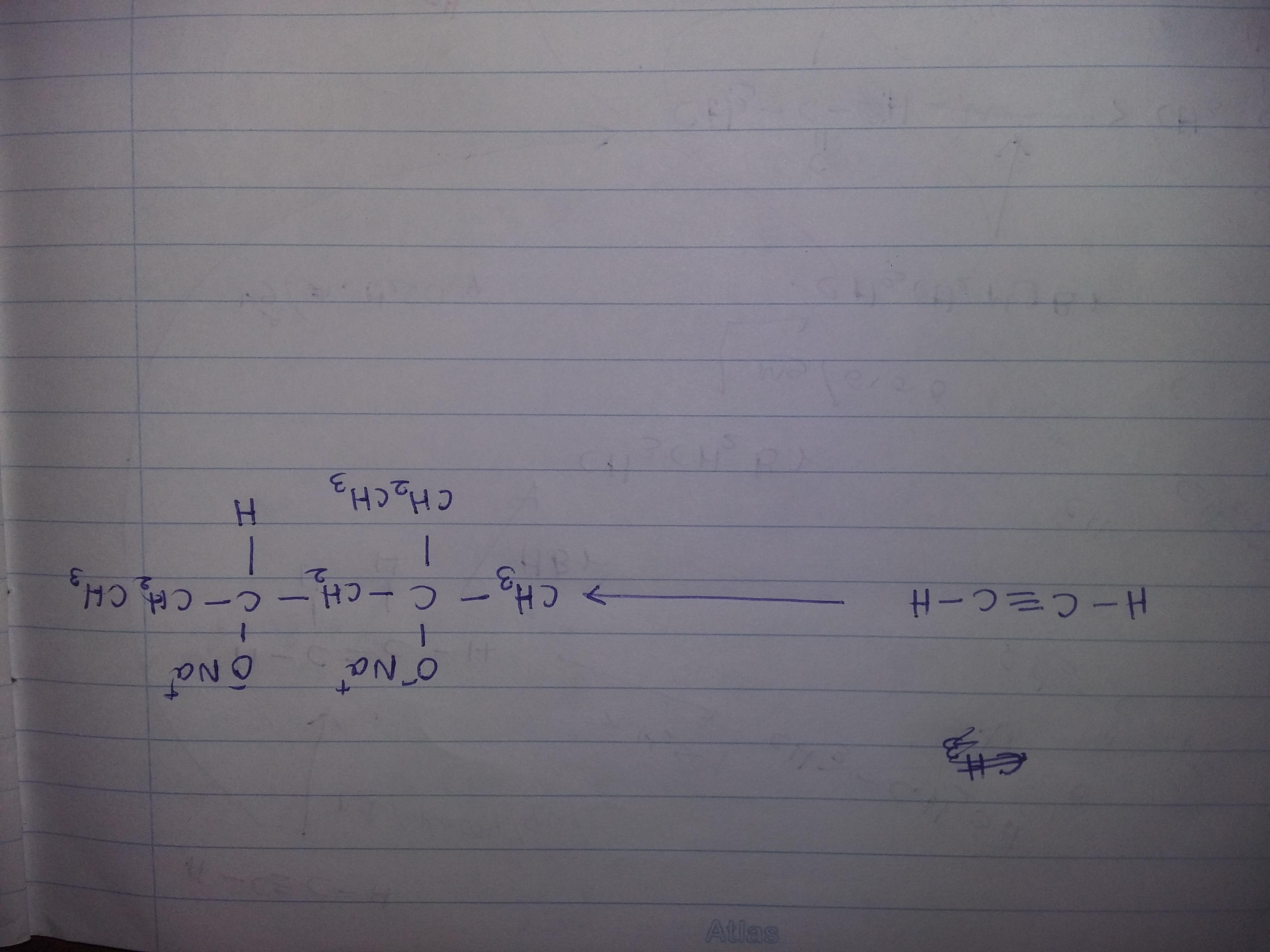
Answers
Organic transformation sequential equation using catalysts will be as follows:
2CH3-CH2-O => (alc. KOH) => CH2=CH2 + KCl + H2O => (Br2/CCl4) => CH2Br-CH2Br + Zn
CH2Br-CH2Br + Zn => (HBr /Pd) => CH2=CH2+ZnBr2
As can be visualized from above organic transformation equation, conversion of dry ether in presence of alkaline potassium hydroxide results in formation of unstable ethene. This dry unsaturated compound of ethene is stabilized by reaction that happens in presence of bromine or calcium tetrachloride as the catalyst which results in formation of ethylene bromide which in presence of highly efficient palladium as catalyst results in formation of stable ethene as byproduct. Thereby with formation of stable compound of ethylene, it releases zinc bromide as byproduct resulting completion of reaction equation. This stable product ethene is a double bonded carbon structure that is chemically extremely flammable and has planar structure.
To know more about organic transformation:
brainly.com/question/14413579
#SPJ1
What is the energy (in J) of a photon with a frequency of 7.49 × 10¹⁴ s⁻¹? (h = 6.626 × 10⁻³⁴ J • s)
Answers
The energy in joules of a photon that has a frequency of 7.49 × 10¹⁴ s⁻¹ is 4.963 × 10-²¹ J.
How to calculate energy?The energy of a photon or light wave can be calculated using the following formula:
E = hf
Where;
E = energy in joulesh = Planck's constant (6.626 × 10-³⁴ J/s)f = frequency (s-¹)According to this question, the frequency a photon is given as 7.49 × 10¹⁴ s⁻¹ where the Planck's constant (h) is 6.626 × 10⁻³⁴ J • s. The energy can be calculated as follows:
E = 7.49 × 10¹⁴ × 6.626 × 10⁻³⁴
E = 4.963 × 10-²¹ J
Therefore, the energy in joules of a photon that has a frequency of 7.49 × 10¹⁴ s⁻¹ is 4.963 × 10-²¹ J.
Learn more about energy of a photon at: https://brainly.com/question/2393994
#SPJ1
Some plants have fleshy stems and leaves that can store water. In which type of habitat would these adaptations be important
1- grassland
2- rainforest
3- mountain
4- desert
Answers
3. Burns from boiling water can be severe, caused by the transfer of energy from the boiling water to the
skin as the water cools to body temperature. How much heat (kJ) is transferred from the from 15.0g
of boiling water at 100.0°C as it hits the skin and cools to 37.2°C?
Answers
Answer:
Q = 3937.56 J
Explanation:
Heat transferred due to change in temperature is given by :
\(Q=mc\Delta T\)
c is the specific heat of water, c=4.18 J/g-°C
We have, m = 15 g, \(T_i=100^{\circ} C\ \text{and}\ T_f=37.2^{\circ} C\)
So,
\(Q=15\times 4.18\times (37.2-100)\\Q=-3937.56\ J\)
Hence, 3937.56 J of heat is transferred.
PLS HELP! WILL GIVE BRAINLIST!
Assume there are 1000 units of energy in the producer level of the energy pyramid. How many units of energy are available at each of the three consumer levels? Show your calculations. Hint first, change the percentages to decimals.
Answers
Answer:
Energy retained at consumer level 1 - units
Energy retained at consumer level 2 - units
Energy retained at consumer level 3 - units
Explanation:
Correctly write the chemical formula for as many ions and compounds as you can:
1. Copper (11) ion
2. Bromide ion
3. Magnesium ion
4. Phosphide ion
5. Copper (11) Bromide
6. Sulfur Dichloride
7. Barium Fluoride
8. Magnesium Phosphate
9. Lithium Permanganate
10. Strontium Sulfite
11. Nitrogen Monoxide
12. Diselenium Tetraoxide
13. Aluminum Sulfide
14. Tin (IV) lodide
15. Beryllium Oxide
16. Potassium Hydroxide
Answers
The chemical formulas for the ions and compounds you listed:
Copper (II) ion: Cu²⁺
Bromide ion: Br⁻
Magnesium ion: Mg²⁺
Phosphide ion: P³⁻
Copper (I) Bromide: CuBr
Sulfur Dichloride: SCl₂
Barium Fluoride: BaF₂
Magnesium Phosphate: Mg₃(PO₄)₂
Lithium Permanganate: LiMnO₄
Strontium Sulfite: SrSO₃
Nitrogen Monoxide: NO
Diselenium Tetraoxide: Se₂O₄
Aluminum Sulfide: Al₂S₃
Tin (IV) Iodide: SnI₄
Beryllium Oxide: BeO
Potassium Hydroxide: KOH
Copper (II) ion: Cu²⁺
Copper (II) ion has a charge of 2+ and is represented by Cu²⁺. This means that copper has lost two electrons, resulting in a 2+ charge.
Bromide ion: Br⁻
The bromide ion has a charge of 1- and is represented by Br⁻. This means that bromine has gained one electron, resulting in a 1- charge.
Magnesium ion: Mg²⁺
The magnesium ion has a charge of 2+ and is represented by Mg²⁺. This means that magnesium has lost two electrons, resulting in a 2+ charge.
Phosphide ion: P³⁻
The phosphide ion has a charge of 3- and is represented by P³⁻. This means that phosphorus has gained three electrons, resulting in a 3- charge.
Copper (I) Bromide: CuBr
Copper (I) bromide is a compound formed by combining copper (I) ion (Cu⁺) and bromide ion (Br⁻). The charges of the ions balance each other, resulting in a neutral compound.
Sulfur Dichloride: SCl₂
Sulfur dichloride is a compound consisting of one sulfur atom (S) and two chlorine atoms (Cl). The subscript "2" indicates the presence of two chlorine atoms.
Barium Fluoride: BaF₂
Barium fluoride is a compound composed of one barium ion (Ba²⁺) and two fluoride ions (F⁻). The charges of the ions balance each other, resulting in a neutral compound.
Magnesium Phosphate: Mg₃(PO₄)₂
Magnesium phosphate is a compound consisting of one magnesium ion (Mg²⁺) and two phosphate ions (PO₄³⁻). The charges of the ions balance each other, resulting in a neutral compound. The subscript "3" indicates the presence of three magnesium ions, and the subscript "2" indicates the presence of two phosphate ions.
Lithium Permanganate: LiMnO₄
Lithium permanganate is a compound composed of one lithium ion (Li⁺) and one permanganate ion (MnO₄⁻). The charges of the ions balance each other, resulting in a neutral compound.
Strontium Sulfite: SrSO₃
Strontium sulfite is a compound consisting of one strontium ion (Sr²⁺) and one sulfite ion (SO₃²⁻). The charges of the ions balance each other, resulting in a neutral compound.
Nitrogen Monoxide: NO
Nitrogen monoxide is a compound composed of one nitrogen atom (N) and one oxygen atom (O). Since the compound does not contain ions, it is represented by its elemental symbols.
Diselenium Tetraoxide: Se₂O₄
Diselenium tetraoxide is a compound consisting of two selenium atoms (Se) and four oxygen atoms (O). The prefix "di-" indicates the presence of two selenium atoms.
Aluminum Sulfide: Al₂S₃
Aluminum sulfide is a compound composed of two aluminum ions (Al³⁺) and three sulfide ions (S²⁻). The charges of the ions balance each other, resulting in a neutral compound. The subscript "
2" indicates the presence of two aluminum ions, and the subscript "3" indicates the presence of three sulfide ions.
Tin (IV) Iodide: SnI₄
Tin (IV) iodide is a compound formed by combining tin (IV) ion (Sn⁴⁺) and iodide ion (I⁻). The charges of the ions balance each other, resulting in a neutral compound.
Beryllium Oxide: BeO
Beryllium oxide is a compound composed of one beryllium ion (Be²⁺) and one oxygen ion (O²⁻). The charges of the ions balance each other, resulting in a neutral compound.
Potassium Hydroxide: KOH
Potassium hydroxide is a compound consisting of one potassium ion (K⁺) and one hydroxide ion (OH⁻). The charges of the ions balance each other, resulting in a neutral compound.
For more question on ions click on
https://brainly.com/question/1310794
#SPJ11
the valency of magnesium is 2 why
Answers
The valency of magnesium is 2 because it has two valence electrons in its outermost shell. Magnesium belongs to the second group of the periodic table, which means it has two electrons in its outermost shell or valence shell. In order to achieve a stable configuration, magnesium tends to lose these two valence electrons to form a positively charged ion with a charge of 2+. This makes magnesium a bivalent or divalent element with a valency of 2.
Answer:
Because the outer shell of magnesium contains 2 atoms
to find the density of stopper I weighted it and found its mass to 4.8g. After that I filled a graduated cylinder with 32.1mL of water. After adding the stopper, the water level rose to 39.2mL. What is the density of the stopper?
Answers
Answer:
0.68g/ml
Explanation:
The density of an object is its mass per unit volume. It is calculated using the formula
Density = mass / volume
Mass of stopper weighed = 4.8g
The volume of stopper can be got by subtracting the (volume of water) from the (volume of water+stopper) i.e.
= 39.2ml - 32.1ml
= 7.1ml
Volume of stopper = 7.1ml
Density of stopper= 4.8/7.1
Density= 0.676056
Therefore, the density of the stopper is 0.68g/ml
Which of the following best describes the arrangement of particles in a gas?
O The particles are spread apart and can move freely.
O The particles are packed closely together and can move freely.
O The particles are packed closely together and cannot move freely
O The particles are spread apart and cannot move freely.
Answers
Answer: The particles are spread apart and can move freely.
What is the rate at which Br⁻(aq) disappears in the reaction below if the rate of disappearance of BrO₃⁻(aq) is 0.041 M/s?
BrO₃⁻ + 5 Br⁻ + 6 H⁺ → 3 Br₂ + 3H₂O
Answers
The rate of disappearance of Br- is 5 times of the rate of disappearance of BrO₃⁻. Hence, the disappearance rate of Br- in the reaction is 0.201 M/s .
What is rate of a reaction ?The rate of a reaction is the rate of disappearance of the reactants or the rate of appearance of the products. The rate of a reaction is directly proportional to the molar concentration of the reactants.
For the given reaction, the rate of reaction can be written in the following terms.
-d/dt [BrO₃⁻] = -1/5 d/dt [Br-] = -1/6d/dt [H+ ] = +1/3d/dt [Br²] = + 1/3 d/dt[H₂O]
The minus sign indicates the disappearance and + indicates the formation.
Given, the rate of disappearance of BrO₃⁻ = -d/dt [BrO₃⁻] = 0.041 M/s.
-d/dt [BrO₃⁻] = 1/5 d/dt [Br-]
then d/dt [Br-] = 5 -d/dt [BrO₃⁻] = 5 × 0.041 M/s = 0.201 M/s.
Therefore, the rate of disappearance of Br- in this reaction is 0.201 M/s.
Find more on reaction rate :
https://brainly.com/question/29261432
#SPJ9
The same mass of 5 different potential fuels was used to heat the same mass of water in a simple calorimeter. The results are shown below. Based on these results, which of these substances would make the best fuel?
Answers
We can see here that the best fuel is the one that produces the most heat per unit mass. In this case, the fuel that produces the most heat per unit mass is methanol.
What is fuel?Fuel is a substance that is used to produce energy through combustion or other chemical reactions. It is commonly utilized to power various forms of transportation, generate heat or electricity, and operate machinery and appliances.
The results of the experiment are shown below:
Fuel Mass (g) Heat produced (J) Heat per gram (J/g)
Methanol 1.0 350 350
Ethanol 1.0 250 250
Propane 1.0 200 200
Butane 1.0 150 150
Pentane 1.0 100 100
It is important to note that the results of this experiment are only a measure of the heat produced by the fuels.
Learn more about fuel on https://brainly.com/question/10172005
#SPJ1
Most ecosystems originally get their energy from
Answers
The sun is bigger than the Moon, but they appear to be the same size in the sky when you look at them from Earth. Why?
O The sun is closer to Earth than the Moon.
O The sun's light reflects off the Moon, making it look the same size as the sun.
O The sun is higher in the sky than the moon.
O The moon is closer to Earth than the sun.
Answers
Answer:
The moon is closer to Earth than the sun! (Last one)
Explanation:
Which term refers to the smallest particle of a compound with all the properties of that compound?
Question 5 options:
mixture
molecule
isotope
nonmetal
Answers
Answer:
molecule. The smallest part of a compound is the molecule. A molecule retains all the properties of that compound.
Explanation:
thanks me later
Use the information in table to find standard enthalpy of the combustion of methane and also create a hess enthalpy diagram for reaction.
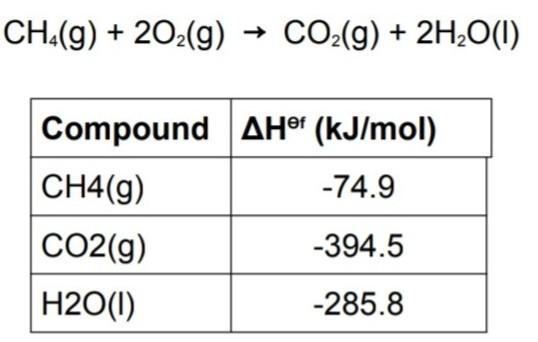
Answers
The combustion of methane can be represented by the equation CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)We are to find the standard enthalpy of combustion of methane.
We are also to create a Hess enthalpy diagram for the reaction. Standard enthalpy of combustion is the amount of energy released when one mole of a substance is completely burned in excess oxygen at standard temperature and pressure. We can use the data in the table to calculate the standard enthalpy of combustion of methane.The table gives us the standard enthalpies of formation of the reactants and products. The standard enthalpy of formation is the amount of energy change that occurs when one mole of a compound is formed from its constituent elements in their standard states (usually at 25°C and 1 atm).We can use the standard enthalpies of formation to calculate the standard enthalpy of combustion of methane. The standard enthalpy of combustion is given by the following equation:ΔH°c = ΣΔH°f(products) – ΣΔH°f(reactants)We can calculate the standard enthalpy of combustion of methane using the standard enthalpies of formation from the table as follows:ΔH°c = [ΔH°f(CO2(g)) + 2ΔH°f(H2O(g))] – [ΔH°f(CH4(g)) + 2ΔH°f(O2(g))]Substituting the values from the table:ΔH°c = [(–393.5 kJ/mol) + 2(–241.8 kJ/mol)] – [(–74.8 kJ/mol) + 2(0 kJ/mol)]ΔH°c = (–890.2 kJ/mol) – (–74.8 kJ/mol)ΔH°c = –815.4 kJ/mol. Therefore, the standard enthalpy of combustion of methane is –815.4 kJ/mol.We can create a Hess enthalpy diagram for the reaction as shown below: The Hess enthalpy diagram is used to show how the standard enthalpy of the reaction can be calculated using the standard enthalpies of formation of the reactants and products. The diagram shows the two steps involved in the reaction. The first step involves the breaking of the bonds in methane and oxygen to form the reactants. The second step involves the formation of the products from the reactants. The standard enthalpy of combustion is the difference between the standard enthalpies of formation of the products and reactants. The Hess enthalpy diagram shows that the same standard enthalpy of combustion can be obtained regardless of the pathway taken to get from reactants to products.
for such more questions on equation
https://brainly.com/question/26694427
#SPJ8
1. in this experiment, why 3-sulfolene was used instead of 1,3-butadiene? explain thoroughly for full credit.
Answers
Starting with solid 3-sulfolene and then breaking it down was simpler than doing it with gaseous 1,3-butadiene. Maleic anhydride, a dienophile, reacts with the diene to produce 4-cyclohexene-cis-dicarboxylic anhydride.
What is sulfolene ?A cyclic organic compound with a sulfone functional group is known as sulfolene or butadiene sulfone. It is a crystalline, odorless, white solid that can be stored forever and dissolves in various organic solvents as well as water. The substance is utilized as a butadiene source.
Sulfolane is a common industrial solvent that is used for cleaning natural gas and extracting aromatic hydrocarbons from hydrocarbon mixtures.
Sulfolane, a dipolar aprotic sulfone solvent, is comparable in physicochemical qualities to other dipolar aprotic solvents as DMSO, NMP, DMF, and DMAC. Sulfolane (anhydrous) has the highest freezing point and highest boiling point among the solvents in Table 1 at 28.4 °C.
Thus, solid 3-sulfolene and then breaking it down was simpler than doing it with gaseous 1,3-butadiene.
To learn more about sulfolene, follow the link;
https://brainly.com/question/29854277
#SPJ1
Including the cis or trans designation, what is the IUPAC name of the following substance?

Answers
Answer:
here's your answerExplanation:
hope it will help you!!!(✿^‿^)
is electrical conductivity a physical property
Answers
Answer:
Yes
Explanation:
I doesn't change the substance or the chemical properties of the substance.
which of the following is a fission reaction?
carbon-12 and hydrogen-1 combining to form a nitrogen-13 atom
Answers
Answer:The reactions are as follows: a carbon-12 (12C) nucleus captures a hydrogen nucleus (1H, a proton) to form a nucleus of nitrogen-13 (13N); a gamma ray (γ) is emitted in the process. The nitrogen-13 nucleus emits a positive electron (positron, e+) and becomes carbon-13 (13C).
an erlenmeyer flask contains 15.00mL of 0.030 M HCI before titration. 5.00 mL of 0.050 of M NaOH is added to the HCI in the flask during titration. What is the mole ratio of acid (HCI) to base (NaOH) in the balanced neutralization equation?
0.05 to 0.03
1:1
1:2
which expression. gives the actual moles of base added?
0.050x5.00
0.030x0.015
0.050x0.005
How many moles of H+ will be present following neautralization?
0.25
0.00025
0.025
Answers
Answer:
1.1
0.050*0.005
0.00025
Explanation: