19. Chemical properties can be used to
(1) differentiate between two compounds
(2) determine the temperature of a substance
(3) determine the density of a substance
(4) differentiate between two neutrons
Answers
Answer:differentiate between two compounds i think!
Explanation: taking a test!
Answer:(1) differentiate between two compounds
Explanation:
A Chemical property tells us the characteristics of a particular matter or substance after a chemical change. This property helps us to differentiate a compound from another or differentiate elements or substances. Examples of a Chemical property are acidity, reactivity and heat, etc .
For example to differentiate between Water H2O and Hydrogen peroxide, H202
Even though they are made up of oxygen and hydrogen. Hydrogen peroxide (H2O2), has more(1) oxygen than water (H2O).
Using Acidity as a chemical property to differentiate these substances
Water, H20 is amphoteric in nature ie having both acidic and basic properties, while H202 is a weak acid.
See related question here: https://brainly.com/question/19594290
Related Questions
Chlorine displaces iodine from a solution of sodium iodide in a redox reaction.
The equation for this reaction is shown.
Cl₂ + 2NaI—>2NaCl + I₂
Which statement about this reaction is correct?
A Chlorine is the oxidising agent and it oxidises iodide ions.
B Chlorine is the oxidising agent and it reduces iodide ions.
C Chlorine is the reducing agent and it oxidises iodide ions.
D Chlorine is the reducing agent and it reduces iodide ions.
Answers
Answer:
(A) chlorine is an oxidizing agent in this reaction so it oxidize iodine and it itself is reduced
Explanation:
Cl2 oxi no. = 0 became Cl- oxi no. = -1
so it is reduced
I- oxi no. = -1 became I2 oxi no. = 0
so it oxidized
How are the environments of a desert and a tundra different?
A. A tundra is at a higher latitude than a desert.
B. A tundra is less humid than a desert.
C. A tundra is much warmer than a desert.
D. A tundra receives less precipitation than a desert.
Answers
Reasoning: Tundra’s experience low temperatures while deserts experience hot/humid temperatures.
chemistry image below

Answers
The two atoms that are of elements in the same group in the Periodic Table are atoms 1 and atom 4.
The correct option is B.
What is common about atoms in the same group in the periodic table?Atoms in the same group in the periodic table share some common characteristics. Here are a few generalizations:
Valence Electrons: Atoms in the same group have the same number of valence electrons. These are the electrons in the outermost shell of the atom that are involved in chemical reactions.Similar Chemical PropertiesSimilar Physical PropertiesThe elements in the same group of the periodic table share some common properties due to their similar electronic configurations.
Leearn more about the periodic table at: https://brainly.com/question/25916838
#SPJ1
What would be the mass in grams of 0.300 moles of the ionic compound formed when magnesium metal reacts with oxygen
Answers
Answer:
Balanced Equation for reaction between Magnesium and Oxygen:
2Mg + O₂ --> 2MgO
Molar Mass of MgO is the atomic masses listed on the periodic table for the two elements Magnesium and Oxygen. Magnesium's molar mass is 24g/mol, and Oxygen's molar mass is 16g/mol. So MgO's molar mass would be 24 + 16 = 40g/mol.
The equation to find moles is:
\(moles \ = \ \frac{mass}{molar \ mass}\)
So if we rearrange this equation to find for mass:
\(mass = moles \ * \ molar \ mass\)
So you have to multiply 0.300 moles by 40, which gives you 12g
Meaning the mass of Magnesium Oxide is 12g.
consider the case . you will see that there are degeneracies in the energy spectrum. some degeneracies have a simple symmetry explanation. if and are degenerate because of this symmetry, what is the relationship between them?
Answers
In the given case, where there are degeneracies in the energy spectrum, some of these degeneracies can be explained by simple symmetry. If states A and B are degenerate due to this symmetry, it implies that there is a relationship between them.
The relationship between the degenerate states A and B can be understood through the concept of symmetry operations. Symmetry operations are transformations that leave the physical properties of a system unchanged. These operations include rotations, translations, and reflections. If states A and B degenerate because of a specific symmetry operation, it means that this operation can transform state A into state B without changing the energy of the system. In other words, the symmetry operation connects the two degenerate states.
The specific relationship between states A and B depends on the nature of the symmetry operation involved. It could be a rotational symmetry, where the states are related by rotation. It could also be a translational symmetry, where the states are related by a translation. Alternatively, it could be a reflection symmetry, where the states are related by a reflection. In summary, if states A and B degenerate due to a symmetry operation, between them is established through that symmetry operation, which can be a rotation, translation, or reflection.
To know more about the energy spectrum please refer:
https://brainly.com/question/29061745
#SPJ11
State with reasons, whether sulphur dioxide is acting as an oxidizing agent or a reducing agent in each of the following reactions:
•2H2S(g) + SO2(g) -> 2H2O(l) + 3S(s)
•SO2(g) +H2O(l) +NaClO(aq) -> NaCl(aq) + H2SO4(aq)
Answers
Answer:
A) oxidizing agent is SO2
B) NaClO is the oxidizing agent
Explanation:
A) This is a redox reaction in which oxidation and reduction occur simultaneously.
Thus, in 2H2S(g) + SO2(g) -> 2H2O(l) + 3S(s);
H2S is reduced as follows;
H2S → S + 2H+ + 2e−
We can see that SO2 has been reduced while H2S gets oxidized since it has changed state from - 2 to 0 . Thus sulphur dioxide is the oxidizing agent.
B) SO2(g) + H2O(l) + NaClO(aq) -> NaCl(aq) + H2SO4(aq)
In this, SO2 undergoes oxidation and NaClO is the oxidizing agent
at the bottom of the ocean a rock has a mass 25 g. At sea level the same rock wilk have a mass of:
less than 25 g
more than 25 g
exactly 25 g
Answers
Answer:
exactly 25 g
Explanation:
The mass of an object is universally the same every where. Even in outer space.
This is because, mass is the amount of matter contained in a substance. Since there is no loss in the amount of matter, the mass of the body stays the same.
It is weight that is subjected to changes with the value of gravity. Weight differs from places to places. A place with higher gravity will have a higher value of weight for any given mass.Mass accounts for every individual matter present in a substance.
When they remain the same, mass does not change. Only a change in the amount of matter in a body can cause variation in mass.
Therefore, at the bottom of the ocean, a body will have the same mass as on top and even at sea level.
Answer:
c. exactly 25 g
Explanation:
The effects of a catalyst on a chemical reaction is to react with the product, effectively removing it and shifting the equilibrium to the right.
(a) True
(b) False
Answers
The statement is false. A catalyst does not react with the product. It increases the rate by lowering activation energy.
The assertion is bogus. An impetus doesn't respond with the result of a compound response. All things being equal, it builds the pace of the response by bringing down the initiation energy expected for the response to happen. This is accomplished by giving an elective response pathway that has a lower enactment energy. Accordingly, more reactants can beat the energy obstruction and respond to frame items, and the harmony is accomplished all the more rapidly. Since the impetus isn't consumed in the response, it doesn't influence the place of harmony, nor does it shift the balance to the right or left. Impetuses are fundamental in numerous modern cycles, as they can speed up the pace of the response and save time and assets.
To learn more about catalyst, refer:
https://brainly.com/question/30651967
#SPJ4
complete the curved arrow pushing mechanism for the following michael (conjugate) addition reaction. add the missing bonds, nonbonding electron pairs, and curved arrows as needed. do not delete any pre‑drawn bonds, charges, or lone pairs. if you accidentally delete a vital part of the structure, use the undo button on the lower left of the drawing canvas. step 1: use curved arrows to show conversion to the next step.
Answers
The full sentences about curved arrow pushing mechanism following michael (conjugate) addition reaction is given below:
Michael (conjugate) addition reaction is during conjugate addition, a nucleophile adds to the electrophilic β-alkene carbon to from a C-Nuc bond.What is a conjugate addition?
A conjugate addition with a carbanion as the nucleophile is described as as the Michael reaction. For the mechanism of this type of process, look at the Michael addition of a simple enolate.
During a Michael reaction the enolate functions as as a nucleophilic donor and the α, β-unsaturated carbonyl does the job as the electrophilic acceptor.
In conclusion, the mechanism is described as a mixture of an alpha-substitution for the enolate and a conjugate addition for the α, β-unsaturated carbonyl.
Learn more about Michael reaction at:
https://brainly.com/question/29768817
#SPJ1
you add iodine to cornstarch solution and produce a new,blue substance
is it a physical change or a chemical change?
Answers
The addition iodine to cornstarch solution and produce a new, blue substance is a chemical change.
What are chemical changes?Chemical changes can be defined as a change of material when two different substance combine to form a new substance with different properties and one or more than one new substance can be formed.
There are basically five type of chemical reactions.
Combination reactionDecomposition reactionDisplacement reactionDouble displacement reactionPrecipitation reactionThus, the addition iodine to cornstarch solution and produce a new, blue substance is a chemical change.
To learn more about chemical change, refer to the link below:
https://brainly.com/question/2591189
#SPJ1
goo bl, kang js, cho sb (2015) treatment of early-stage erythematotelangiectatic rosacea with a q-switched 595-nm nd:yag laser. j cosmet laser ther 17(3):139–142
Answers
The article "Treatment of early-stage erythematotelangiectatic rosacea with a Q-switched 595-nm Nd:YAG laser" by Goo BL, Kang JS, Cho SB (2015) focuses on the use of a specific laser, the Q-switched 595-nm Nd:YAG laser, for treating early-stage erythematotelangiectatic rosacea.
The study is published in the Journal of Cosmetic and Laser Therapy, volume 17, issue 3, pages 139-142. The mentioned article investigates the effectiveness of using a Q-switched 595-nm Nd:YAG laser in the treatment of early-stage erythematotelangiectatic rosacea, a common skin condition characterized by facial redness and visible blood vessels. The study aims to evaluate the outcomes of this laser treatment modality.
The Q-switched 595-nm Nd:YAG laser is a specific type of laser that emits light at a wavelength of 595 nanometers (nm). This particular wavelength is targeted at the blood vessels and redness associated with rosacea, aiming to reduce the visible signs and symptoms.
The authors likely conducted a clinical study involving patients with early-stage erythematotelangiectatic rosacea and assessed the efficacy of the laser treatment. The article may include details on the treatment protocol, the number of sessions, the outcomes observed, and any side effects or complications reported.
Overall, this study contributes to the understanding of the use of the Q-switched 595-nm Nd:YAG laser as a potential treatment option for early-stage erythematotelangiectatic rosacea, providing insights into its effectiveness and potential benefits for patients with this condition.
To learn more about rosacea, here
https://brainly.com/question/31631139
#SPJ4
Mention and discuss briefly the adverse effects of chemistry.
Answers
Depending on the chemical, these longer-term health effects might include:
organ damage. weakening of the immune system. development of allergies or asthma. reproductive problems and birth defects. effects on the mental, intellectual or physical development of children. cancer.2. There is a toxic spill in Birch Bay. The material has a half-life of 3 days. What is the daily decay rate of the substance? Do not just give an answer. Show all work and any equations you used to find your answer. Round your answer to 3 decimal places
Answers
The daily decay rate of the substance is approximately 0.793 (rounded to 3 decimal places).
The half-life of the material = 3 days To calculate:
The daily decay rate of the substance Formula used:
1/2^(t/h), where t = time elapsed and h = half-life of the substance Solution:
The formula for calculating the daily decay rate of the substance is given by:
1/2^(t/h)Where t is the time elapsed and h is the half-life of the substance.
The daily decay rate of the substance is calculated as follows:1/2^(1/3) ≈ 0.793.
To know more about decay rate please refer to:
https://brainly.com/question/30068164
#SPJ11
The pH of an aqueous solution of hydrochloric acid is 2. What is the pH of the solution after the addition of 10 g of sodium chloride?
Answers
Answer:
wala ako alam sagot sorry
if you flirt with someone else is that considered cheating ?
Answers
Answer:
Depends how your flirting
Explanation:
Answer:
yes, because if you love someone you wont want anyone else. therefore if you flirt your giving someone else the attention your significant other is suppose to get from you.
Explanation:
an effervescent tablet is added to a test tube of water, producing a solution that fizzes and releases gas bubbles. The total mass of the substances and the test tube were recorded before and after the tablet was added to the test tube of water.
Answers
Answer:
The mass remains same after and before the reaction
Explanation:
This is because of law of conservation of mass
Which states that
In a chemical reaction mass is neither created nor destroyed,it remains conserved .LINKS WILL BE REPORTED PLEASE HELP
Certain bacteria live and grow on the roots of some plants and produce chemicals that are beneficial to the plants. Which of the following observations best supports the claim that this relationship is beneficial to the plants?
A.When the bacteria are removed from the plant roots and are grown in a laboratory setting, they fail to survive
B.The population size of the bacteria varies greatly depending on the chemistry of the soil and the type of the plant
C.Plants with a higher density of the bacteria on their roots have increased rates of survival and reproduction
D.The chemical produced by the bacteria can be created by humans and added to the soil where it reaches the roots of the plant
Answers
C. the denser the plants the better.
Answer:
C
Answer: Plants with higher density of the bacteria on their roots have increased rates of survival reproduction
Explanation:
This is really easy plz help me out 25 points

Answers
2. Rain water harvesting helps collect rain water.
3. Use of recycled materials eg construction waste or wood can help in reducing waste.
4. Proper tree plantation can help to keep the environment cool and give shade.
5. Using alternative materials such as bamboo or earth construction can also reduce carbon footprints.
6. Proper windows can provide enough daylight in house and hence reduce electricity.
What is a polysaccharide and what are the differences between the plant polysaccharides?
Answers
Polysaccharide is a type of carbohydrate (such as glycogen, cellulose, or starch) whose molecules are made up of many sugar molecules bound together.
What purpose does the polysaccharide serve?
In general, polysaccharides serve one of two purposes: they either store energy or sustain structural integrity. Highly compact polymers like starch and glycogen are employed to store energy. In plants and animals, cellulose and chitin, two linear polymers, provide structural support.
What is plant polysaccharide?
More than half of the carbs we consume come from starch, which is the most significant source of carbohydrates in the human diet. Granules of it can be found in plants. Amylose and amylopectin, two polymers, are combined to form starch. 10%–30% amylose and 70%–90% amylopectin make up natural starches.
Learn more about the Polysaccharides with the help of the given link:
https://brainly.com/question/780562
#SPJ4
An element with a mass number of 11 and an atomic number of 5 has how many
neutrons?
Answers
Answer:
6 neutrons
Explanation:
6 neutrons
Boron having an atomic number of 5 means that it will have 5 protons. 11 atomic mass units in total. Neutrons also have a atomic mass unit of 1. So there are 6 neutrons
1. What amount of ammonia (in moles) is produced by the reaction of 4.00 mol H2 with 3.00 mol Nz?
3 H2(g) + N2(g) → 2 NH3(g)
Answers
3 H₂ + N₂ → 2 NH₃
↓ ↓
4 mol 3 mol
Since the moles of N₂ is the smaller of the two reactants, then N₂ is the limiting factor (the reactant that will decide how much ammonia is produced since it has the smaller amount of moles). ∴ we have to use it in calculating the number of moles of ammonia
The mole ratio of N₂ to NH₃ based on the balanced equation is 1 to 2.
∴ the moles of NH₃ = moles of N₂ × 2
= 3 moles × 2
= 6 moles
What is sodium fluoride?
Answers
Answer:
Sodium fluoride is a colorless crystalline solid or white powder, or the solid dissolved in a liquid. It is soluble in water. It is noncombustible.(I got this answer from g o o g l e)
Explanation:
Explain how steam causes burns by describing the transfer of heat.
Answers
Answer:
It will be : (in simple terms)
Condensation of hot steam or hot vapor on the cooler skin releases the water's latent heat of vaporization, rapidly raising the temperature of the skin which will result in steam burns.
Hope it helped
All the best!!
Answer the following questions: On a 10-fold dilution of a weak acid, the pH will ______________ . On a 10-fold dilution of a weak base, the pH will ______________. If one adds a very small amount of strong base to a buffered solution, the pH will _______________. Can you make a buffer using a strong acid
Answers
Answer:
i) increase
ii) decrease
iii) remain the same
iv) No, because it dissociates completely.
Explanation:
On a 10-fold dilution of a weak acid, the pH will increase because the concentration of hydrogen ions will decrease thereby increasing the pH to close to that of water.
On a 10-fold dilution of a weak base, the pH will decrease due to the removal of hydroxide ions from the solution. This results in the solution having a H closer to that of water.
If one adds a very small amount of strong base to a buffered solution, the pH will remain constant because a buffer solution acts to withstand any change to its pH on the addition of small quantities of either an acid or a base.
A buffer solution cannot be made with a strong acid because thy undergo complete dissociation. Therefore, any small addition of base or acid will result in very large changes in the pH of the solution. A buffer solution is made with a weak acid and its conjugate base or a weak base and its conjugate acid.
how much energy must be transferred as heat for a reversible isothermal expansion of an ideal gas at 99°c if the entropy of the gas increases by 25.6 j/k?
Answers
The amount of energy that must be transferred as heat for a reversible isothermal expansion of an ideal gas is equal to the change in entropy multiplied by the temperature of the gas. Therefore, for the reversible isothermal expansion of an ideal gas at 99°C, the energy that must be transferred as heat is 25.6 J/K x 99°C = 2532.4 J.
Here, correct answer will be
This energy is necessary for the expansion to occur reversibly, meaning that the temperature of the gas remains constant and the entropy increases.
This energy is used to overcome the intermolecular forces that would normally resist the expansion of the gas, allowing it to expand reversibly and increase its entropy.
Know more about isothermal expansion here
https://brainly.com/question/4674503#
#SPJ11
Why shouldn’t we fill up balloons with hydrogen gas ?
Answers
Answer: Because hydrogen is a gas.
Explanation: If children were running around with balloons filled with hydrogen gas, then they would catch fire. So the answer to your question is, its simply dangerous.
The hydrogen emission spectrum is shown below. What is the energy of the
410 nm emission line? (The speed of light in a vacuum is 3.00 x 108 m/s, and
Planck's constant is 6.626 x 10-34 J.s.)
400
750 pm

Answers
Answer:
C.) 4.85 x 10⁻¹⁹ J
Explanation:
To find the energy, you need to use the following equation:
E = hc / w
In this formula,
-----> E = energy (J)
-----> h = Planck's Constant (6.626 x 10⁻³⁴ J*s)
-----> c = speed of light (3.00 x 10⁸ m/s)
-----> w = wavelength (m)
Once you have converted nanometers to meters, you can plug the given values into the equation and solve.
410 nm 1 m
------------- x ---------------------- = 4.10 x 10⁻⁷ m
1 x 10⁹ nm
E = hc / w
E = (6.626 x 10⁻³⁴ J*s)(3.00 x 10⁸ m/s) / (4.10 x 10⁻⁷ m)
E = 4.85 x 10⁻¹⁹ J
Someone help pleaseeee I will give brainliest
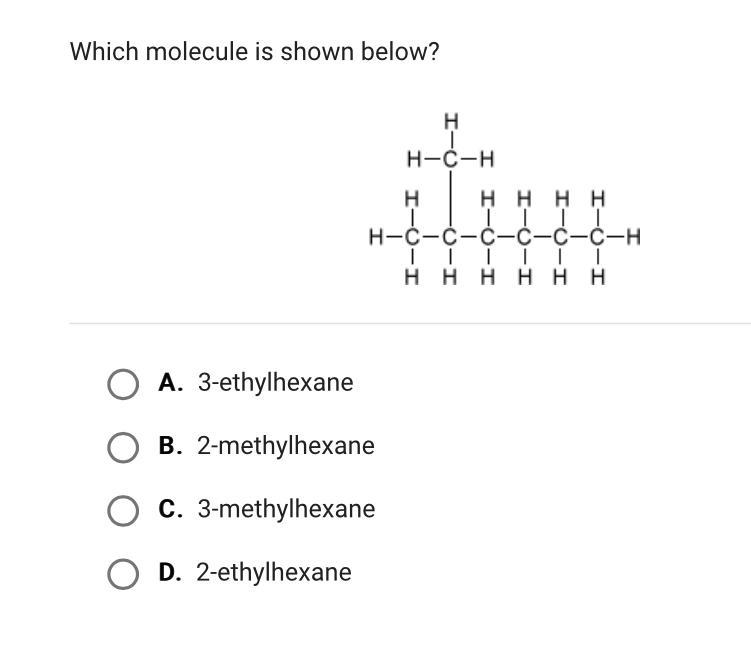
Answers
Answer:
2-Methylhexane
Because Ethylexane does not have hydrogen molecules neither,also it only has 2 options but they're just repeated
Determine the number of atoms of O in 7. 23 moles of Ca(NO₃)₂
Answers
The number of atoms of Oxygen (O) in 7. 23 moles of Ca(NO₃)₂ = 2.61 × 10²⁵ atoms.
To determine the number of atoms of O in 7.23 moles of Ca(NO₃)₂, we need to use the formula for the number of atoms, which is:
Number of atoms = Number of moles × Avogadro's number × Number of atoms in one molecule
Avogadro's number is 6.022 × 10²³ atoms/mole.
In one molecule of Ca(NO₃)₂, there are 6 atoms of O.
So, the number of atoms of O in 7.23 moles of Ca(NO₃)₂ is:
Number of atoms of O = 7.23 moles × 6.022 × 10²³ atoms/mole × 6 atoms/molecule
= 2.61 × 10²⁵ atoms of O
Therefore, there are 2.61 × 10²⁵ atoms of O in 7.23 moles of Ca(NO₃)₂.
Learn more about atomic number here: https://brainly.com/question/29749207
#SPJ11
11.All of the following properties of a diamond are physical except...Select one:a. It does not conduct electricity.b. It produces carbon dioxide when burned in pure oxygen.c. It is transparent like glass.d. It is the hardest material.
Answers
Answer
b. It produces carbon dioxide when burned in pure oxygen.
Explanation
The reaction between diamond and oxygen, producing carbon dioxide is not a physical property of diamond, it is a chemical property because breaking and synthesis of chemical bonds occur.