10. How many moles of CuCl, are in 150 L of a 2.33 M Cuci, solution?
Answers
Answer:
349.5 moles
Explanation:
(I am assuming that there is a typo at the end, where Cuci solution = CuCl solution)
The formula for molarity is:
Molarity = moles in solution / volume of solution
Here, we are given both the Molarity and the volume. So we can plug it into the equation above and get:
2.33 = moles / 150
Now we solve for the moles by doing:
moles = 2.33 * 150
This gives us the answer of 349.5 moles.
Related Questions
An olympic bronze medal is made up of three elements, copper, zinc and tin. State the number of types of atom in the medal.
Answers
An athlete who finishes third in any sport receives an Olympic bronze medal. Copper, zinc, and tin are the three elements that make up an Olympic bronze medal.
What does metal consist of?The Olympic Games are the top sporting event in the globe. There are three types of medals an athlete can win in a sport. These include the first-, second-, and third-place prizes of gold, silver, and bronze, respectively.
The second-place finisher in each sport receives a bronze medal. These are constructed of copper, zinc, tin, and very little silver. Each bronze medal used in the Olympics had 1% silver and the remaining 99% was bronze alloy used in coinage
The composition of bronze coins is 97% copper, 2.5% zinc, and 0.5% tin.
Here is further information on medals:
brainly.com/question/28181336
#SPJ2
How many moles (of molecules or formula units) are in each sample?
A) 32.66 g CF2Cl2
B) 24.2 kg Fe (NO3) 2
C) 0.5912 g C8H18
D) 104 kg CaO
Express all answers to 4 sig figs
Answers
Answer:
A = 1.627 ×10²³ molecules of CF₂Cl₂.
B = 809.4 ×10²³ molecules of Fe(NO₃)₂.
C = 0.03117 ×10²³ molecules of C₈H₁₈.
D= 1116 ×10²³ molecules of CaO.
Explanation:
A)
Given data:
Mass of CF₂Cl₂ = 32.66 g
Number of moles = ?
Number of molecules = ?
Solution:
Number of moles = mass/ molar mass
Number of moles = 32.66 g/ 120.9 g/mol
Number of moles = 0.2701 mol
Number of molecules:
1 mole = 6.022×10²³ molecules
0.2701 mol×6.022×10²³ molecules / 1mol = 1.627 ×10²³ molecules of CF₂Cl₂.
B) Given data:
Mass of Fe(NO₃)₂ = 24.2 Kg (24.2×1000 = 24200 g)
Number of moles = ?
Number of molecules = ?
Solution:
Number of moles = mass/ molar mass
Number of moles = 24200 g/ 180 g/mol
Number of moles = 134.4 mol
Number of molecules:
1 mole = 6.022×10²³ molecules
134.4 mol×6.022×10²³ molecules / 1mol =809.4 ×10²³ molecules of Fe(NO₃)₂.
C) Given data:
Mass of C₈H₁₈= 0.5912 g
Number of moles = ?
Number of molecules = ?
Solution:
Number of moles = mass/ molar mass
Number of moles = 0.5912 g/ 114.22 g/mol
Number of moles = 0.005176 mol
Number of molecules:
1 mole = 6.022×10²³ molecules
0.005176 mol×6.022×10²³ molecules / 1mol =0.03117 ×10²³ molecules of C₈H₁₈.
D) Given data:
Mass of CaO = 104 Kg (104×1000 = 104,000 g)
Number of moles = ?
Number of molecules = ?
Solution:
Number of moles = mass/ molar mass
Number of moles = 104,000 g/ 56.1 g/mol
Number of moles = 1854 mol
Number of molecules:
1 mole = 6.022×10²³ molecules
1854 mol×6.022×10²³ molecules / 1mol = 1116 ×10²³ molecules of CaO.
Use the drop-down menus to answer the questions.
As the amount of surface area increases, what happens to the rate of this reaction?
If the antacid tablet were broken into eight pieces, what would be a reasonable estimate for the time of reaction?
Answers
Answer:
the rate is faster, and the time of reaction is 28 seconds
Explanation:
i did the test
Answer:
The rate is faster, 28 seconds
Explanation:
A cup sits on a table. Due to its position, the potential energy of the cup is 3.00 joules. Ignoring frictional effects, if the cup falls off the table, how much kinetic energy will it have when it is halfway to the floor? a) 3.00 J b) 1.50 J c) 0.00 J d) cannot be determined without knowing the mass and the height of the cups
Answers
Answer: 1.50 J
Explanation:
Answer:
1.5 joules
Explanation:
Due to conservation of energy, half way the potential energy will be 1.5J so the remaining 1.5J is kinetic energy.
Which substance, when mixed with water, will produce the best conductor of
electricity?
a) table salt
b) granulated sugar
c) carbon dioxide
d) motor oil
Answers
Answer: a) Table Salt
good luck
How does the position of a metal in the periodic table relate to whether it occurs primarily as an oxide or as a sulfide?
Answers
The elements from the left side of the P.T are found as oxides and right side are found as sulfides.
Where are oxides found in periodic table ?Metals towards the left side of the periodic table have lower ionization energies. Metals towards the left side of the periodic table have lower electro negativities they tend to give up electrons. The O2- ion is small cation closely which has high lattice energy for the oxides.
Therefore, the elements from the left side of the P.T are found as oxides.
Where are sulfides found in periodic table ?Metals towards the right side of the periodic table have higher ionization energies. Metals towards the right side of the periodic table have higher electro negativities they tend to form covalent bonds which suits the larger, more polarizable (S2-) ion.
Therefore, the elements from right side of the P.T are found as sulfides.
Thus from the above conclusion we can say that The elements from the left side of the P.T are found as oxides and right side are found as sulfides.
Learn more about the Periodic Table here: https://brainly.com/question/15987580
#SPJ4
A burning candle is an example of a chemical reaction taking place. What can be inferred about this reaction?
A. There are reactants but no products.
B. The reaction absorbs energy.
C. The reaction releases energy.
D. Mass is not conserved.
Answers
Answer:C
Explanation:
Explaination:
what are four states, or phrases of matter? describe the shape and volume properties of each phase, can they change or are they fixed?
Answers
Answer:
solid liquid gas and plasma
How many grams of oxygen are needed to react with excess methane in the
following reaction, in order to produce 325 kJ of energy?
Answers
23.7 grams of oxygen are needed to react with excess methane in order to produce 325 kJ of energy.
To answer this question, we first need to determine the balanced chemical equation for the reaction between methane and oxygen: CH₄+ 2O₂ → CO₂ + 2H₂O + 890 kJ
From this equation, we can see that for every mole of methane (CH₄) that reacts, we get 890 kJ of energy released. However, the question is asking for the amount of oxygen (O₂) needed to produce a specific amount of energy (325 kJ).
To solve for the amount of oxygen needed, we can use stoichiometry. We know that the reaction produces 890 kJ of energy per mole of methane reacted, so we can set up a proportion:
890 kJ/1 mol CH₄ = 325 kJ/x mol CH₄
Solving for x gives us:
x = (325 kJ)(1 mol CH₄)/(890 kJ) ≈ 0.37 mol CH₄
So we need 0.37 moles of methane to produce 325 kJ of energy. Now we can use the balanced equation to determine how many moles of oxygen we need:
1 mol CH₄ requires 2 mol O₂
Therefore, we need:
0.37 mol CH₄ × (2 mol O2/1 mol CH₄) = 0.74 mol O₂
Finally, we can convert moles of oxygen to grams using the molar mass of oxygen (32 g/mol):
0.74 mol O₂ × (32 g/mol) = 23.7 g O₂
So, 23.7 grams of oxygen are needed to react with excess methane in order to produce 325 kJ of energy.
To know more about methane, refer
https://brainly.com/question/25649765
#SPJ11
(e) A 0.050 mol sample of a hydrocarbon was burned in excess oxygen.
The products were 3.60 g of water and 6.60 g of carbon dioxide.
(i) Calculate the number of moles of carbon dioxide produced.
Relative atomic masses: C = 12; O = 16.
Moles of carbon dioxide =
*
(2)
Answers
The correct answer is 0.15.
We are aware that there is 0.05 mol of an unidentified hydrocarbon we will refer to as "X" and that its burning produces 6.6 g of carbon dioxide and 3.6 g of water.
These quantities might be converted to moles by applying the following formula:
amount= mass/ relative atomic mass
Thus, the following equation may be written for H2O: moles = 3.6 / 18 = 0.2 and for CO2: moles = 6.6 / 44 = 0.15.
0.05X + x'O2 = 0.15CO2 + 0.2H2O
This may be made simpler by dividing through by 0.05 (this step is likely to be the most helpful to you), resulting in:
1 x + x O2 = 3 co2 + 4 H2O
The hydrocarbon must have been the source of all the carbon in the carbon dioxide and all the hydrogen in the water.
Accordingly, 4 x 2 = 8 moles of H and 3 x 1 = 3 moles of C.
There are 3/1 = 3 Cs and 8/1 = 8 Hs in one X molecule.
This clearly identifies C3H8 or propane as the hydrocarbon X (dividing by 1 seems unnecessary, but it illustrates the process to use if there were more than one mol of X in the first equation).
To learn more about number of moles of carbon dioxide refer the link:
https://brainly.com/question/12723070
#SPJ9
look carefully at the three reactions you have performed. what is the mole ratio between copper in reaction 1 and the copper
Answers
The mole ratio between copper in reaction 1 and the copper in reaction 2 and 3 is 1:1.
The mole ratio represents the proportion of the amounts of two substances in a chemical reaction, measured in moles.
Mole ratio is commonly used in stoichiometric calculations in chemistry, which helps to balance equations and to predict the amount of reactants needed to produce a particular product.
In the three reactions that have been performed, the mole ratio between copper in reaction 1 and the copper in reaction 2 and 3 is 1:1.
This means that the amount of copper present in reaction 1 is equal to the amount of copper present in reactions 2 and 3.
To learn more about mole ratio, visit:
https://brainly.com/question/30851942
#SPJ11
Questlon 5 of 5
What is the main source of energy for plants?
A. Oxygen
B. Water
C. Soil
D. Sunlight
Answers
Answer:
D Sunlight
Explanation:
Hoped that helped :)
Violet light has a frequency of 7.31 *10^19 Hz. What is the wavelength?
Answers
The frequency of violet light is 7.31 * 10¹⁹ Hz. The wavelength of violet light is 2.43 * 10¹¹
What is wavelength?The formula of wavelength is fλ = c,
f= 7.31 * 10¹⁹ Hz.
c= 3 * 10⁸ m/s
fλ = c
7.31 * 10¹⁹ Hz. * λ = 3 * 10⁸ m/s
7.31 * 10¹⁹ Hz./3 * 10⁸ m/s = λ
2.43 * 10¹¹ = λ
Therefore, The frequency of violet light is 7.31 * 10¹⁹ Hz. The wavelength of violet light is 2.43 * 10¹¹.
To learn more about wavelength, refer to the link:
https://brainly.com/question/13533093
#SPJ1
pollutants from a factory that enter the water in a watershed will have a negative effect on which of the following

Answers
Answer:
a
Explanation:
The pollution will kill the local pond life, and amphibians are very delicate to change.
Mark me brainliest?
The pollutants from a factory will have a negative effect on
a. local pond life
This effect is known as water pollution.
Effects of water pollution on local pond life:The plants go through such a lot of oxygen during the evening and during rotting processes that there is none left for the other lake life. The development additionally forestalls daylight arriving at the life forms beneath. At last, all the green growth bites the dust leaving a rancid, rotting mass.
Natural matter and supplements cause an expansion in high-impact green growth and exhaust oxygen from the water section.
Thus, correct option is a.
Find more information about Water pollution here:
brainly.com/question/24623157
A heat sensing resistor that changes its value as its temperature changes is known as a:_____.
Answers
A heat sensing resistor that changes its value as its temperature changes is known as a Thermistor.
The Thermistor is a special type of variable resistive detail that adjustments its physical resistance while uncovered to modifications in temperature. The Thermistor is a solid kingdom temperature sensing device which acts a chunk like an electrical resistor however is temperature touchy. A thermistor is a temperature sensitive resistor. they may be regularly used as a temperature sensor.
Thermocouples are the maximum generally used form of temperature sensor. they are used in business, automotive, and purchaser programs. Thermocouples are self-powered, require no excitation, can function over a extensive temperature variety, and feature brief response times.
Learn more about Thermistor here:-https://brainly.com/question/14531016
#SPJ4
Explain Redox tiltration and examples of half - life reactions.
Answers
Answer:
Explanation:
Half life reactions take place in electrochemistry where u have to find the emf generated from a cell
E.g Mg(s)/Mg(aq)//Ag(aq)/Ag(s)
A cross results in three phenotypes. A nual typothesis of recessive epistasis is tested. Your calculated chi-square value is 6.4. Assuming a p Value of 0.05, do you reject the null frypothesis? yes. No
Answers
The null hypothesis is rejected when the chi-square value is higher than the critical value or p-value. In this case, the chi-square value of 6.4 is higher than the critical value for a p-value of 0.05, which is 3.84. Therefore, the null hypothesis is rejected.
A cross results in three phenotypes. A null hypothesis of recessive epistasis is tested. Your calculated chi-square value is 6.4. Assuming a p-value of 0.05, do you reject the null hypothesis?The answer is yes.What is a chi-square value?A chi-square value is a measure of how different two sets of data are. It compares the observed data to the expected data and generates a statistic that shows how far apart they are. It compares the observed data to the expected data and generates a statistic that shows how far apart they are. The chi-square test is a statistical hypothesis test that is used to determine if there is a significant difference between two groups of data. The null hypothesis is rejected when the chi-square value is higher than the critical value or p-value. In this case, the chi-square value of 6.4 is higher than the critical value for a p-value of 0.05, which is 3.84. Therefore, the null hypothesis is rejected.
To know more about hypothesis visit:
https://brainly.com/question/32562440
#SPJ11
write and balance the equation showing the reaction between iron (iii) oxide and carbon monoxide to form iron (ii) oxide and carbon oxide. sum the coefficients
Answers
The balanced chemical equation showing the reaction between iron (iii) oxide and carbon monoxide to form iron (ii) oxide and carbon oxide is :
FE2O3 + 3CO → 2FE +3CO2
Unbalanced:
Fe2O3 + CO → Fe + CO2
The atoms of iron on the reactant side is 2 but on the product side is 1, so have to change the coefficient of the iron of the product side. Place 3 with the CO in reactants and 3 with CO2 on the product side.
A balanced chemical equation is defined as the number of atoms of each type in the reaction being equal to the reactants and product sides.
The mass and charge is equal in a balanced chemical reaction.
If you need to learn more about the balanced chemical equation, click here
brainly.com/question/28294176?referrer=searchResults
#SPJ4
Is CuS an ionic, non polar or polar covalent bond and why
Answers
Answer:
Ionic
Explanation:
Answer:
Ionic Bond
Explanation:
CuS has an ionic bond between it as it it formed by the combination of two ions Cu⁺² and S⁻².
the natural cements that hold clasts together precipitate in the empty pore spaces after compaction. those precipitates come from
Answers
Natural cements that hold clasts together precipitate in the empty pore spaces after compaction due to the combined effects of;
Pressure, temperature, chemical composition, and length of exposure to groundwater. The most common cements are calcite, quartz, clay, and iron oxide.
The natural cements that hold clasts together precipitate in the empty pore spaces after compaction come from the groundwater that has traveled through the sediment.
The groundwater carries dissolved ions of calcium, silica, and other elements.
As the pore spaces are compressed, the pressure on the water within them increases, causing the dissolved ions to come out of solution as solid minerals or precipitates.
These cements act to bind the sediment particles together and are commonly composed of calcite, quartz, clay, and iron oxide.
Compaction of the sediment is one of the major factors controlling cementation. As the sediment is buried deeper and pressure increases, the pore spaces are squeezed shut, forcing the fluids out of them.
Once the water is squeezed out, the dissolved ions can come out of solution and cement the sediment together.
Temperature is another factor that influences the rate of cementation. The warmer the temperature, the faster the reactions occur, so cementation can be accelerated at higher temperatures.
In addition to temperature and pressure, the chemical composition of the groundwater also affects cementation.
The concentration of ions in the water determines what cements are formed, as the most soluble ions will be precipitated first.
For example, calcium ions in groundwater will be the first to precipitate, forming calcite cement.
Finally, the length of time during which the sediment is exposed to the groundwater can also influence cementation. The longer the sediment is exposed to the water, the more time it has to react and form cement.
to know more about cement refer here:
https://brainly.com/question/15286339#
#SPJ11
name the process Wich take place when .a solid carbon lv dry ice changes directly into gas
Answers
Answer:
evaporation takes place
2 Al + Fe2O3 = Al2O3 + 2 Fe
If 0.915 mol Al2O3 is produced, how many grams of iron is produced?
Answers
Answer:
−7.389056fo3+5.436564f
−l2o3+2l
Explanation:
2al+f(2.7182822)o3=al2o3+2f(2.718282)
Step 1: Add -al^2o^3 to both sides.
7.389056fo3+2al+−al2o3=al2o3+5.436564f+−al2o3
−al2o3+7.389056fo3+2al=5.436564f
Step 2: Add -7.389056fo^3 to both sides.
−al2o3+7.389056fo3+2al+−7.389056fo3=5.436564f+−7.389056fo3
−al2o3+0fo3+2al=−7.389056fo3+5.436564f
Step 3: Factor out variable a.
a(−l2o3+2l)=−7.389056fo3+5.436564f
Step 4: Divide both sides by -l^2o^3+2l.
a(−l2o3+2l)
−l2o3+2l
=
−7.389056fo3+5.436564f
−l2o3+2l
a=
−7.389056fo3+5.436564f
−l2o3+2l
Someone help me out with this brain pop ⚠️
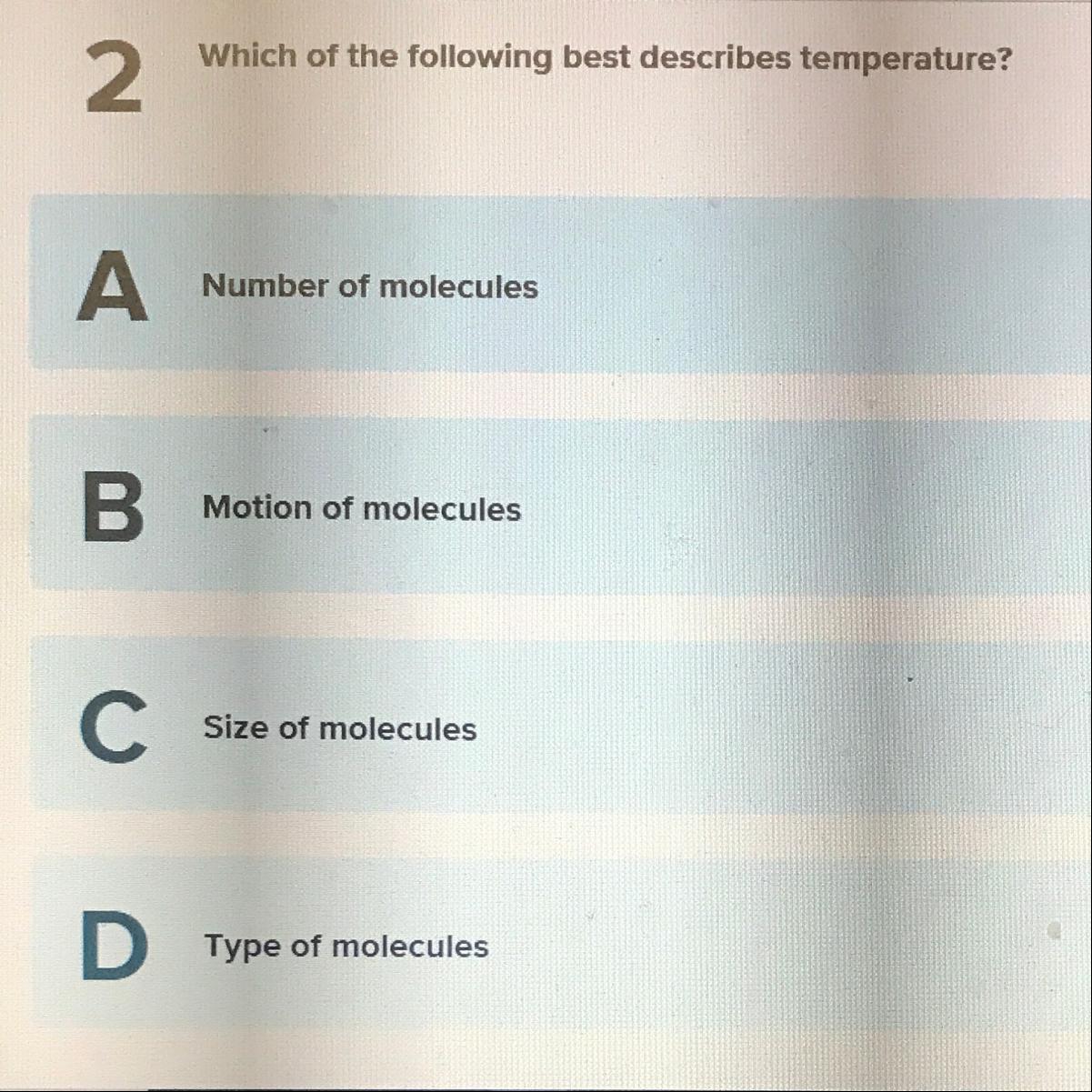
Answers
Imagine that you have taken a sample of water from a nearby lake. You do a test and find that it has
a pH of 5.6. Adding about of a teaspoon of baking soda to it neutralizes it. What does this tell you
about the baking soda?
Answers
The acidic or alkaline character of a solution is obtained from the value of pH. Here baking soda is a base which neutralizes the acid.
What is pH?The pH of a solution is defined as the negative logarithm of hydronium ion concentration in moles per litre. It can be expressed as:
pH = -log [H₃O⁺]
For acidic solution, the pH will be less than 7 and for basic solution the pH will be greater than 7.
Here the sample of water which has the pH of 5.6 is acidic and it is neutralized by the base baking soda.
Thus we can say that baking soda is a base.
To know more about pH, visit;
https://brainly.com/question/27945512
#SPJ9
Which of the following best describes weight?
The amount of space an object takes up
The amount of matter in an object
The force on an object due to its mass and gravity
The force on an object due to its speed and location
(Trying To Help My Sister ion member how 2 do ts)
Answers
Answer:
What best describes the concept of weight is the force on an object due to its mass and gravity.
Explanation:
Weight is the force with which gravity pulls a body or object towards the earth. It is a physical unit that involves both the force of gravity and the mass that an object has.
In general, the terms mass and weight are different, being the mass a characteristic of the body -without influence of other factors- and the weight is the force with which the gravity pushes the mass of that same body.
Regarding the other options:
The amount of space an object takes up , corresponds to the volume of a body.The amount of matter in an object , corresponds to the mass.The force on an object due to its speed and location, is related to the movement of a body.which indicator of those you used would you expect to be useful in determining the ph of a 4.0 x 10^-4 m hcl solution, why?
Answers
One indicator that would be useful in determining the pH of a 4.0 x 10^-4 M HCl solution is phenolphthalein. This indicator changes color in a pH range of 8.2 to 10.0, which is outside the pH range of strong acids like HCl.
Therefore, the solution would remain colorless when phenolphthalein is added. This indicates that the solution is acidic and has a pH below 8.2. Alternatively, a pH meter or a universal indicator could also be used to determine the pH of the solution. However, phenolphthalein is a more suitable choice because it specifically indicates the presence of acidic or basic conditions and eliminates the need for calibration, unlike a pH meter. To determine the pH of a 4.0 x 10^-4 M HCl solution, you would expect a pH indicator with a transition range around pH 3 to 4 to be useful. This is because HCl is a strong acid and will dissociate completely in water, producing H+ ions.
The concentration of H+ ions in the solution will determine its pH. Using the formula pH = -log[H+], you can calculate the pH to be approximately 3.4. An appropriate indicator for this pH range would be methyl orange, which has a transition range from 3.1 to 4.4 and changes color from red to yellow as the pH increases.
To know about phenolphthalein:
brainly.com/question/30675188
#SPJ11
Even at moderate temperatures, attractions between molecules seem to have little effect on the elasticity of gas-gas collisions because:_______.
A) They do affect molecular behaviour and we generally ignore it.
B) Collisions are so brief, and molecules moving so fast, the attractions have insufficient time to significantly affect the system.
C) Strong attractions do have an impact, but the randomness of the collisions cancel those attractions out.
D) Collisions between molecules are so rare, intermolecular forces have no measurable impact.
Answers
Even at moderate temperatures, attractions between molecules seem to have little effect on the elasticity of gas-gas collisions is as a result of Choice B: Collisions are so brief, and molecules moving so fast, the attractions have insufficient time to significantly affect the system.
As a result of the high kinetic energy of gases; the movement of gases is so fast and randomised.
As such, during gas-gas collisions; The collisions are so brief and molecules moving so fast, the attractions have insufficient time to significantly affect the system.
Read more;
https://brainly.com/question/19449287
(04.01 LC)
Fill in the blank with the correct number to balance the equation: __KCIO3 - 2KCI +
302. (Enter only a whole number.) (3 points)
Answers
2, 2, 3 (coefficients)
experiment 1: calculate the combined mass of the two reactants: hydrochloric acid and sodium hydroxide
Answers
The combined mass of hydrochloric acid and sodium hydroxide is determined by adding their individual masses.
When calculating the combined mass of hydrochloric acid and sodium hydroxide, we need to consider the individual masses of these two substances. Hydrochloric acid (HCl) has a molecular formula of HCl and consists of one hydrogen atom (H) and one chlorine atom (Cl). Sodium hydroxide (NaOH), on the other hand, is composed of one sodium atom (Na), one oxygen atom (O), and one hydrogen atom (H). To calculate the combined mass, we add the individual masses of these reactants.
The molar mass of hydrogen (H) is approximately 1 gram/mol, while the molar mass of chlorine (Cl) is approximately 35.5 grams/mol. Sodium (Na) has a molar mass of around 23 grams/mol, oxygen (O) has a molar mass of approximately 16 grams/mol, and hydrogen (H) has a molar mass of around 1 gram/mol.
To determine the combined mass of hydrochloric acid and sodium hydroxide, we multiply the number of atoms of each element by their respective molar masses and sum them up. For example, hydrochloric acid has one hydrogen atom and one chlorine atom, so the total mass would be 1 gram/mol (hydrogen) + 35.5 grams/mol (chlorine). Similarly, sodium hydroxide has one sodium atom, one oxygen atom, and one hydrogen atom, resulting in a combined mass of 23 grams/mol (sodium) + 16 grams/mol (oxygen) + 1 gram/mol (hydrogen).
Learn more about hydrochloric acid
https://brainly.com/question/1451933
#SPJ11
1. 8g of magnesium were added to 60cm³ of 2.1M aluminium sulphate solution in a beaker and stirred gently with a thermometer. The temperature of the mixture rose from 25.0°C to 32.0°C. (Specific heat capacity = 4.2KJKg"K"). Apart from temperature change, state any other observation made. be observed as the displacement (b) (c) Effervescence may (1 mark) hydrogen gou u дей Produced during reaction Calculate the molar enthalpy of displacement of aluminium by the magnesium.(2 marks) Give the thermo ionic equation for the reaction. (1 mark)
Answers
The thermo ionic equation for the reaction is:
3Mg(s) + 2Al₂(SO₄)₃(aq) → 3MgSO₄(aq) + 2Al(s)
Step by step explanationFrom the given information, we can calculate the amount of heat energy released during the displacement reaction of aluminium by magnesium:
Heat energy released = mass of solution × specific heat capacity × temperature change
The mass of the solution can be calculated as follows:
mass of solution = volume of solution × density of solution
= 60 cm³ × 1.04 g/cm³
= 62.4 g
The moles of aluminium sulphate in the solution can be calculated as follows:
moles of Al₂(SO₄)₃ = molarity × volume of solution
= 2.1 mol/L × 0.06 L
= 0.126 mol
Since the reaction is 3Mg + 2Al₂(SO₄)₃ → 3MgSO₄ + 2Al, we know that 2 moles of aluminium are displaced by 3 moles of magnesium. Therefore, the moles of magnesium that reacted can be calculated as follows:
moles of Mg = (0.126 mol Al₂(SO₄)₃ ÷ 2) × (3 ÷ 2) = 0.189 mol
The molar enthalpy change can be calculated as follows:
ΔH = heat energy released ÷ moles of Mg
= (62.4 g × 4.2 J/gK × 7 K) ÷ 0.189 mol
= -583.6 kJ/mol
(note the negative sign indicating an exothermic reaction)
Apart from the temperature change, effervescence may be observed due to the production of hydrogen gas.
The thermo ionic equation for the reaction is:
3Mg(s) + 2Al₂(SO₄)₃(aq) → 3MgSO₄(aq) + 2Al(s)
Learn more about enthalpy here https://brainly.com/question/12356758
#SPJ1