Answers
Answer:
1. Dehydration of alcohols
2. Dehydrohalogenation of alkyl halides
3. Decarboxylation of carboxylic acids
4. Pyrolysis of esters
5. Deamination of amino acids
6. Dealkylation of ethers
7. Dehalogenation of aryl halides
8. Dehydration of amides
9. Dehydrogenation of alkanes
10. Dehydrogenation of alkenes.
Explanation:
Related Questions
What is the other product for this reaction ? H3PO4 + Ca(OH)2 —> H20 + _________
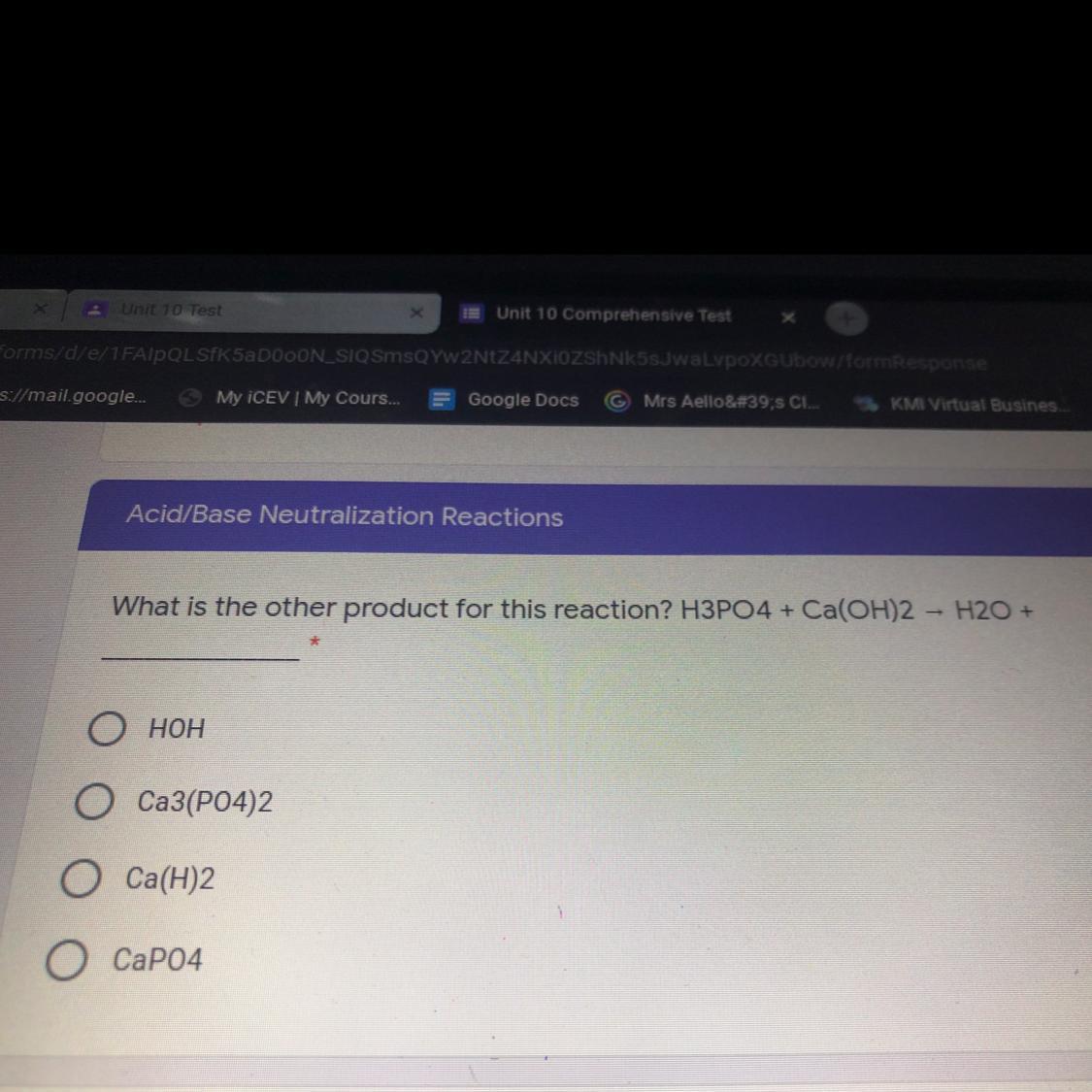
Answers
*☆*――*☆*――*☆*――*☆*――*☆*――*☆*――*☆*――*☆**☆*――*☆*――*☆*――*☆
Answer: h3po4 + ca(oh)2 = h2o + ca3(po4)2
Explanation:
I hope this helped!
<!> Brainliest is appreciated! <!>
- Zack Slocum
*☆*――*☆*――*☆*――*☆*――*☆*――*☆*――*☆*――*☆**☆*――*☆*――*☆*――*☆
Determine the pH of a 3.4x10^-6 M solution of HNO3

Answers
Answer: pH of the solution is 5.47
Explanation: Since HNO3 is a strong acid the concentration of H+ is the same as NO3- only because they are in 1-to-1 ratio. Both ions will have 3.4 x 10^-6 M.
The formula of pH is: pH = -log( the equilibrium concentration of H+ )
When you plug in the concentration of H+:
pH = -log (3.4 x 10^.6 M)
pH = 5.47
Remember: Only the digits after the decimal point are significant figures in logarithms.
For example: 5.47 only has 2 significant figures.
Justification of Subaquatic soil if it is sediment or soil (on the point of view of a geologist)
Answers
Subaquatic soil can be classified as sediment or soil based on its geological properties and formation processes.
Sediment refers to any material that is transported and deposited by water, wind, ice, or gravity. Sediments can be composed of various materials, such as minerals, rocks, organic matter, and even human-made debris.
Sediments can accumulate in different environments, such as rivers, lakes, oceans, and deserts, and can be deposited in layers over time.
Subaquatic soil can be classified as sediment or soil based on its geological properties and formation processes. If it has primarily formed through sediment deposition, it is more appropriate to classify it as sediment.
To learn more about the subaquatic soil, follow the link:
https://brainly.com/question/29471454
#SPJ1
would you rather eat pizza the rest of your life, or cake?
Answers
Answer:
cake
Explanation:
now here me out, no cake is specified so i can say i want a cake that has pizza on it, take off the pizza, and eat it, this goes with any other food without me having to eat the cake
4. Circle the element with the greatest atomic radius. [4]
C.
a.
sodium or magnesium
b. magnesium or beryllium
lithium or rubidium
d. cesium or radon
oxygen or fluorine
f. phosphorus or aluminum
g. calcium or barium
h. boron or gallium
e.
Answers
The atomic greatest radius is magnisium
A chemical reaction in which electrons are transferred from one atom to another is a(n)
Answers
A chemical reaction in which electrons are transferred from one atom to another is an ionic bond.
What do you mean by the term an ionic bond ?The total transfer of certain electrons from one atom to another results in the formation of an ionic bond.
A negatively charged ion known as a cation results from an atom losing one or more electrons. An anion, an ion with a negative charge, is created when an atom gains one or more electrons.
Ionic bonds are formed for a number of reasons, one of which being the stark disparities in electronegativity between atoms, which draw other atoms near them for the exchange of electrons.
Thus,A chemical reaction in which electrons are transferred from one atom to another is an ionic bond.
To learn more about an ionic bond, follow the link;
https://brainly.com/question/11527546
#SPJ1
Starting with 0.3500 mol CO(g) and 0.05500 mol COCl2(g) in a 3.050 L flask at 668 K, how many moles of CI2(g) will be present at equilibrium?
CO(g) + Cl2(g)》COCl2(g)
Kc= 1.2 x 10^3 at 668 K
Answers
At equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
1: Write the balanced chemical equation:
\(C_O\)(g) + \(Cl_2\)(g) ⟶ \(C_OCl_2\)(g)
2: Set up an ICE table to track the changes in moles of the substances involved in the reaction.
Initial:
\(C_O\)(g) = 0.3500 mol
\(Cl_2\)(g) = 0.05500 mol
\(C_OCl_2\)(g) = 0 mol
Change:
\(C_O\)(g) = -x
\(Cl_2\)(g) = -x
\(C_OCl_2\)(g) = +x
Equilibrium:
\(C_O\)(g) = 0.3500 - x mol
\(Cl_2\)(g) = 0.05500 - x mol
\(C_OCl_2\)(g) = x mol
3: Write the expression for the equilibrium constant (Kc) using the concentrations of the species involved:
Kc = [\(C_OCl_2\)(g)] / [\(C_O\)(g)] * [\(Cl_2\)(g)]
4: Substitute the given equilibrium constant (Kc) value into the expression:
1.2 x \(10^3\) = x / (0.3500 - x) * (0.05500 - x)
5: Solve the equation for x. Rearrange the equation to obtain a quadratic equation:
1.2 x \(10^3\) * (0.3500 - x) * (0.05500 - x) = x
6: Simplify and solve the quadratic equation. This can be done by multiplying out the terms, rearranging the equation to standard quadratic form, and then using the quadratic formula.
7: After solving the quadratic equation, you will find two possible values for x. However, since the number of moles cannot be negative, we discard the negative solution.
8: The positive value of x represents the number of moles of \(Cl_2\)(g) at equilibrium. Substitute the value of x into the expression for \(Cl_2\)(g):
\(Cl_2\)(g) = 0.05500 - x
9: Calculate the value of \(Cl_2\)(g) at equilibrium:
\(Cl_2\)(g) = 0.05500 - x
\(Cl_2\)(g) = 0.05500 - (positive value of x)
10: Calculate the final value of \(Cl_2\) (g) at equilibrium to get the answer.
Therefore, at equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
For more such questions on equilibrium, click on:
https://brainly.com/question/517289
#SPJ8
help please help please help please

Answers
4. The process which leads to transition from the above task are as follows:
Ocean - Atmosphere: Evaporation Atmosphere - Clouds: Condensation Clouds - Snow: Condensation Glacier ( river ice ) - River: Melting Cloud - Soil: Precipitation5. The two processes that cause transition from each given below:
Ocean - Cloud is: Evaporation and transpiration Cloud - Glacier is: Evaporation and precipitation6. The major reason why water shortages are the problems for many people around the world is simply because of climatic change which alters the weather of a given place at a period of time, thereby leading to water insufficiency.
What is meant by melting?Melting refers to a chance of state of matter which refers to the process whereby a solid substance changes to liquid.
From the context of the above task, river ice melts into river in a process known as melting.
In conclusion, we can now deduce from the explanation given above that transition is a process which involves the change in form of matter.
Read more on melting:
https://brainly.com/question/40140
#SPJ1
moles of each product that would form as a result of the decomposition of aspirin
Answers
The decomposition of aspirin (acetylsalicylic acid,\(C_{9} H_{8} O_{4}\)) can occur through the hydrolysis reaction, resulting in the formation of acetic acid (\(CH_{3} COOH\)) and salicylic acid (\(C_{7} H_{6}O_{3}\)).
The decomposition of aspirin (acetylsalicylic acid, \(C_{9} H_{8} O_{4}\)) can occur through the hydrolysis reaction, resulting in the formation of acetic acid (\(CH_{3} COOH\)) and salicylic acid (\(C_{7} H_{6}O_{3}\)). To determine the moles of each product formed, we need to consider the balanced chemical equation for the reaction:
\(C_{9} H_{8} O_{4} = > C_{7} H_{6}O_{3} +CH_{3} COOH\)
From the equation, we can see that for every 1 mole of aspirin, 1 mole of salicylic acid and 1 mole of acetic acid are produced.
Therefore, the moles of salicylic acid and acetic acid formed will be equal to the number of moles of aspirin that decomposes. If we know the amount of aspirin in moles, we can directly calculate the moles of each product based on stoichiometry.
For more question on aspirin
https://brainly.com/question/25794846
#SPJ8
1)Grignard reagent when reacted with methanol will yield A) ethanol (B) secondary alcohols (C) tertiary alcohols (D ropanol (E) primary alcohol
Answers
When the reaction of Grignard reagent reacted with methanol will yield a tertiary alcohol. Therefore, Option C tertiary alcohol is correct.
Contains a carbon-metal link, Grignard reagents are chemicals used in catalysis. They generally result from the anhydrous reaction of magnesium metal with an alkyl or aryl halide. Because of their high reactivity, Grignard reagents frequently act as nucleophiles in organic reactions.
An alkyl group from a Grignard reagent binds to the oxygen atom of methanol (CH3OH) when it interacts with the methanol, breaking the carbon-metal connection. A precursor alkoxide is created as a result. The equivalent alcohol is then produced by protonating the intermediate alkoxide.
The reaction of a Grignard reagent with methanol leads to the formation of a tertiary alcohol.
Learn more about reagents here:
https://brainly.com/question/29729676
Unit 4.2 quiz chemistry

Answers
Answer:
H₂SO₄ is the correct answer sorry im late
Which electron configuration is impossible?
a. ls22s22p2d2
b. 1s22s22p63s23p64s
c. 1s22s22p63s23p3
d. 1s22s22p63s2
e. 1s22s22p63s23s54s
1
Answers
Answer:
MP hippopotamus
Explanation: I'm in the fifth grade so yeah I really don't know this so I'm just going to say some random stuff and white cheddar mac and cheese warm TV perfect back MD 11443 to 2885 eleven 12:20 to 11 132 0.24 answer
Bromine trifluoride’s free-energy change is –229.4 kJ/mol, and water vapor’s is –228.6 kJ/mol. Though their free-energy changes are almost the same, bromine trifluoride reacts violently with water vapor, releasing much more energy than the water vapor. The field of chemistry called ______________ explains why bromine trifluoride reacts one way and water vapor reacts another during a reaction.
Answers
The field of chemistry that explains why bromine trifluoride reacts differently than water vapor during a reaction is called chemical kinetics.
What is Energy?
Energy is a fundamental concept in physics, often defined as the ability to do work. It can take many different forms, including thermal energy, kinetic energy, potential energy, electromagnetic radiation, and chemical energy, among others. Energy can be transferred from one system to another, and it can also be converted from one form to another. The SI unit of energy is the joule (J), although other units such as the calorie and the electronvolt are also used in specific contexts.
The field of chemistry that explains why bromine trifluoride reacts differently than water vapor during a reaction is called reaction kinetics. Reaction kinetics is the study of how fast chemical reactions occur and what factors affect the reaction rate. It takes into account factors such as the nature of the reactants, concentration, temperature, surface area, and the presence of a catalyst or inhibitor.
Learn more about Energy
https://brainly.com/question/2003548
#SPJ1
m A 10.00g sample of a substance is found to contain 5.12g of water. What is the percent by of water in the compound? A
. 5.12% B. 4.88% C. 48.8% D. 51.2%
Answers
Answer:
The answer is D) 51.2%
Explanation:
5.12g of 10.00g of the substance is water:
percentage of water= 5.12 x 10.00 =51.2%
Answer:
b
Explanation:
Rose rides horses, and she works at the barn to pay for her riding lessons. When Rose is cleaning stalls at the barn, it takes two full wheelbarrows for her to clean three stalls. Assuming partial wheelbarrows are possible, how many wheelbarrows will it take for Rose to clean 14 stalls?
Answers
Answer:
its d
Explanation:
i got it right
It is given that, 2 full wheel barrows are required for cleaning of 3 stalls. Then about 9 and half wheel barrows are required to clean 14 stalls.
What is wheel barrows?Wheel barrows are metallic containers with three wheels attached within. Wheel barrows are mainly used to transport materials across short distances. It needs to pull or push across the way. The handle in front helps to hold it.
The weight of materials that can be transported using the barrows depends on the volume of barrows.
It is given that, rose needs two full wheel burrows. The partial wheel burrows are also possible.
Then, the number of wheel burrows required to clean 14 stalls is calculated as follows:
2 × 14 / 3 = 9.3.
Therefore, 9 full wheelbarrows and one half burrow is needed to clean 14 stalls.
Find more on wheelbarrows:
https://brainly.com/question/12220011
#SPJ2
Determine the amount of heat required to vaporize 75.0 mL of acetone (C3H6O) at 25 oC. The heat of vaporization (kJ/mol) at 25oC is 31.0. The density and the molar mass of acetone is 0.791 g/mL and 58.08 g/mol, respectively.
Answers
The amount of heat required to vaporize 75.0 mL of acetone at 25°C is 31.651 kJ.
First, we need to determine the mass of acetone that is being vaporized: mass = density * volume
= 0.791 g/mL * 75.0 mL
= 59.33 g
Next, we can use the molar mass of acetone to determine the number of moles of acetone
moles = mass / molar mass
= 59.33 g / 58.08 g/mol
= 1.021 mol
The heat required to vaporize this amount of acetone can be calculated using the heat of vaporization
heat = moles x heat of vaporization
heat = 1.021 mol x 31.0 kJ/mol = 31.651 kJ
To know more about heat:
https://brainly.com/question/13761513
#SPJ1
Americans drink 5,604,000 gallons of sweet tea each day. How many liters is
that? (1 gal = 3.79 L)
Answers
5604000gallon =21239160 liters
Which of the following is an example of sp3 d2 hybridization?
A. C₂H6
B. PC15
C. 1F7
D. SFO
Answers
A cook had a jar containing a sweet food and a jar containing a sour food. The image above shows the sweet and sour foods. At room temperature, both foods are liquids. The same amount of energy was transferred into both substances. Later, one of the foods had changed phase while the other had not. Which food changed phase, and how did it change? PLEASE ANSWER ASAP
Answers
Answer: One of the foods was sour and one was sweet
Explanation:
The sweet one has changed because it has more sugar then the sour one, It either got moldy or melted.
most of the volume of an atom is occupied by ?

Answers
G. Empty space
Hope this helps!
How does nitrogen in the atmosphere become beneficial to plants and eventually animals?
O Nitrogen must be mixed in the soil by earthworms,
O Nitrogen must be converted into a useful form by bacteria,
O Nitrogen must come in contact with decaying organic material,
O Nitrogen must come in contact with the ground through rainfall
Answers
Answer:
O Nitrogen must be converted into a useful form by bacteria.
This bacteria is called the nitrogen fixing bacteria such as the Rhizobium bacteria.
Part c moles of base in antacid sample
Mole base In antacid/mass of antacid sample
Average molole base in antacid/mass of antacid sample

Answers
An antacid tablet's CaCO content should be between 25 and 35% by mass.
To completely neutralise 20 ml of HCl solution, 19.85 ml of 0.01 M NaOH solution is required.
How many moles of NaOH are required for neutralisation?It is a neutralisation reaction in which sodium hydroxide, a basic, interacts with to form salt sodium phosphate, which is salt and water. According to the stoichiometric mole ratio, three moles of sodium hydroxide are needed to neutralise one mole of phosphoric acid.
To calculate the amount of moles of acid neutralised by the tablet, subtract the number of moles of acid neutralised in the titration from the starting solution's moles of acid.
learn more about antacid tablet
https://brainly.com/question/27720401
#SPJ1
Determine how many moles of Ca (OH) 2 are required to completely neutralize 3.26 mol of HC2H3O2.
Answers
Moles of Ca (OH)₂ required to completely neutralize 3.26 mol of HC₂H₃O₂ : 1.63
Further explanationA reaction coefficient is a number in the chemical formula of a substance involved in the reaction equation. The reaction coefficient is useful for equalizing reagents and products.
Reaction
2HC₂H₃O₂ + Ca(OH)₂ → Ca(C₂H₃O₂)₂ + 2H₂O
mol of HC₂H₃O₂ = 3.6
From equation above, mol ratio of HC₂H₃O₂ : Ca(OH)₂ = 2 : 1, so mol Ca(OH)₂ :
\(\tt mol~Ca(OH)_2=\dfrac{1}{2}\times 3.26=1.63\)
The vaporization of Br2 from the liquid to the gas state requires 7.4 kcal/mol. write a reaction showing heat as a product or reactant
Answers
Answer:
Br₂(l) + ΔV → Br₂(g)
Explanation:
When a chemical or phase change occurs during a chemical process some heat is absorbed or released. For the process of vaporization of a substance, the heat (Usually required) is ΔV (How many energy is required for the process occurs).
In the vaporization of Br2 there are required 7.4kcal/mol, Δv. The reaction is:
Br₂(l) + ΔV → Br₂(g)Which of the following carbocations is(are) likely to undergo a rearrangement?
A. only I
B. I and III
C. II and III
D. I, II, III

Answers
The cations that are more likely to undergo rearrangement are II and III Option C
Why does cations undergo rearrangement?
Cation rearrangement is a phenomena that causes cations, which are positively charged ions, to reorganize themselves under specific conditions. The term "cation rearrangement " describes the movement of ions inside a substance or solution that is caused by a variety of elements, including concentration gradients, electric fields, or chemical reactions.
Rearrangements influence the mobility and redistribution of cations inside substances or solutions, which can have a significant impact on the system's behavior and characteristics.
Learn more about cation rearrangement:https://brainly.com/question/32195409
#SPJ1
A bag of potato chips is sealed in a factory near seal level. The atmospheric pressure is 99.82 kPa. What is the difference in Pa between the pressure in the bag and the atmospheric pressure?
Answers
The difference in Pa between the pressure in the bag and the atmospheric pressure is 1.505 kPa.
How to obtain the difference in pressureTo obtain the difference in pressure, we first need to know the atmospheric pressure near sea level. This is 760 mm Hg. When we convert this to pascals, we will have, 101.32472 kPa.
Now, the difference in pressure will be obtained by subtracting the atmospheric pressure in the bag from the atmospheric pressure near sea level and this is:
101.32472 kPa - 99.82 kPa
= 1.505 kPa.
Learn more about pressure here;
https://brainly.com/question/28012687
#SPJ1
Aluminum can be made by reducing alumina Al2O3 by carbon in the reaction equation
2 Al2O3 + 3 C → 4 Al + 3 CO2
according to. How much carbon is needed to reduce Al2O3 to produce 491 grams of pure aluminum? To give an answer to the gram, be sure to add the unit g after the numerical value of your answer.
Answers
Taking into account the reaction stoichiometry, 163.67 grams of C is needed to reduce Al₂O₃ to produce 491 grams of pure aluminum.
Reaction stoichiometryIn first place, the balanced reaction is:
2 Al₂O₃ + 3 C → 4 Al + 3 CO₂
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
Al₂O₃: 2 molesC: 3 molesAl: 4 molesCO₂: 3 molesThe molar mass of the compounds is:
Al₂O₃: 102 g/moleC: 12 g/moleAl: 27 g/moleCO₂: 44 g/moleThen, by reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
Al₂O₃: 2 moles ×102 g/mole= 204 gramsC: 3 moles ×12 g/mole= 36 gramsAl: 4 moles×27 g/mole= 108 gramsCO₂: 3 moles×44 g/mole= 132 gramsMass of carbon requiredThe following rule of three can be applied: if 108 grams of Al are produced by 36 grams of C, 491 grams of Al are produced by how much mass of C?
mass of C= (491 grams of Al× 36 grams of C)÷ 108 grams of Al
mass of C= 163.67 grams
Finally, 163.67 grams of C is required.
Learn more about the reaction stoichiometry:
brainly.com/question/23035393
brainly.com/question/24741074
brainly.com/question/21683406
brainly.com/question/24653699
#SPJ1
What type of bond will form between nitrogen and bromine?
Answers
Answer:
Non-polar covalent bond
Explanation:
The bond between two nonmetal atoms is always covalent.
hope my answer helps you

How many grams are presented in a 7.4 moles sample of H2O?
Answers
Answer:
130g
Explanation:
For every mole of a substance, there is a molar mass that coincides with the masses on the periodic table. For one mole of H2O, you would add the molar masses of the two hydrogen atoms and the oxygen atom to get 18.016g. You would then take this molar mass and multiple it by the amount of moles present (7.4) to get 133.3184g
From here, you would round to the significant figures to get 130g
Someone help with these questions??

Answers
Answer:
2) true 3)I think I A and there you go