Answers
Answer:
D
Explanation:
The Phoenicians contributed to ocean exploration by establishing the first trade routes throughout the Mediterranean, even as far north as Great Britain.
Related Questions
Photoelectric effect will occur only if frequency of light striking an electron in a metal is above a certain threshold frequenci
Answers
The statement is correct. The photoelectric effect refers to the phenomenon where electrons are ejected from the surface of a material when it is exposed to light. The frequency of light striking an electron in a metal must be above the threshold frequency in order for the photoelectric effect to occur.
The statement is correct. The photoelectric effect refers to the phenomenon where electrons are ejected from the surface of a material when it is exposed to light. However, for the photoelectric effect to occur, the frequency of the incident light must be above a certain threshold frequency.
The threshold frequency is the minimum frequency of light required to dislodge electrons from the material. Below this threshold frequency, regardless of the intensity or duration of the light, no electrons will be emitted.
This behavior can be explained by the particle-like nature of light, where light is composed of discrete packets of energy called photons. The energy of a photon is directly proportional to its frequency. Only photons with energy greater than or equal to the binding energy of the electrons in the material can dislodge them.
Therefore, the frequency of light striking an electron in a metal must be above the threshold frequency in order for the photoelectric effect to occur.
For more question on photoelectric
https://brainly.com/question/1458544
#SPJ8
what is the answers to this someone pls help

Answers
Answer:
The nuclide formed by the β decay of 26Al is 26Mg.
Mark my answer as brainliest! this was a difficult one
what would happen to an atom in its ground state of external energy was applied to the electrons g
Answers
Answer:
See explanation
Explanation:
According to Bohr's theory, when external energy is supplied to an atom, its electrons absorb energy and move from a lower energy level to a higher energy level.They quickly return to their original level and re-radiate the absorbed energy as a photo of light.
Hence, when an atom in ground state receives external energy, it becomes excited because energy is transferred to its electrons and they move from lower to higher energy level.
The electron after getting the energy would jump to an orbital further away from the nucleus.
• Ground state refers to the lowest energy state an atom can be at. When an electron in an atom captivates energy it is considered to be in an excited state.
• An excited atom becomes unstable and seem to align itself to move back to its lowest energy state.
• When an atom in its ground state is supplied with an external energy, the energy is absorbed by the atom and the electron becomes excited and jumps to an orbit , that is, distant from the nucleus.
• It can be said that electron attains higher energy state, with the absorption of more energy by the atom, the electron further moves to higher state till it becomes a free electron and no longer remains the part of the atom.
Thus, the electron after getting the energy would jump to an orbital further away from the nucleus.
To know more about:
https://brainly.com/question/13525116
If 3.0 grams of Strontium-90 in a rock sample remained in 1989, approximately how many grams of Strontium-90 were present in the original sample in 1933?
Answers
11.29 grams of strontium-ninety had been gift withinside the unique rock pattern in 1933. Strontium- ninety decays to yttrium-ninety, which in flip decays to solid zirconium.
The isotopes of strontium and yttrium emit beta debris as they decay. The launch of radiation throughout this decay manner reasons situation approximately the protection of strontium and all different radioactive substances. Sr-ninety may be inhaled, however ingestion in meals and water is the best fitness situation. Once withinside the body, Sr-ninety acts like calcium and is effectively included into bones and teeth, wherein it may purpose cancers of the bone, bone marrow, and smooth tissues across the bone.
Strontium has a half-existence of 28.8 years. Therefore,
1989 - 1933 = 56years.
fifty six / 28.eight = 1.94 half-lives
Thus, the amount of the radioisotope closing will double for every half-existence elapsed. transferring backwards in time. Therefore, transferring backwards in time through 1.94 times half-lives, the amount closing will double through 1.94 times.
Thus, the quantity closing in 1933 is 3.0 × (1.ninety four)² = 11.29 grams.
Learn more about Strontium-90 visit: brainly.com/question/14351021
#SPJ4
I need the math to it to show how I got to the answer, please help #2

Answers
The number of moles of the water produces by the 1.3 moles of O₂ is 1.00 mol of H₂O. The correct option is C.
The chemical equation is as :
2C₄H₁₀ + 13O₂ ----> 8CO₂ + 10H₂O
The moles of the O₂ = 1.3 moles H₂O
The molar mass of the O₂ = 32 g/mol
2 moles of the C₄H₁₀ produces the 10 moles of the water,
13 moles of O₂ produces the 10 moles of H₂O
The moles of the H₂O = (10/13) × 1.3
The moles of the H₂O = 1 mol
The number of moles of the water is 1 mol of the H₂O and the moles of the oxygen that is O₂ is the 1.3 moles.. Therefore, the correct option is C.
To learn more about moles here
https://brainly.com/question/31597231
#SPJ1
True or false questions?
Most metal elements are brittle and do not conduct electricity. True or False
Any element can be identified by counting the protons in its neucleus. True or False
All Earths matter is made from the combination of about 100 elements. True or false
Answers
Answer:
1. False 2.True3.True? Unsure
Explanation:
A 10g metallic block measures has a volume of 5 cm^3. What is the density of the metallic block in g/cm^3
Answers
A 10g metallic block measures has a volume of 5 cm^3. The density of the metallic block in g/cm^3 is 2gm/cm^3. The density of a metallic block is calculated by p=m/v
What, for example, is density?Its density is the amount of "stuff" that can be packed into a given amount of space. A block of the tougher, lighter element gold (Au), for example, will have a higher density than a block of the heavier element lead (Pb).
Calculation of the density of the above problem.The density of a substance is given by the following formula:~
ρ = m/v
where the respective alphabets stands for
ρ is the density
m is the mass
V is the volume
by replacing in formula :
p = 10(gm)/5cm^3
p = 2gm/cm^3
To know more about Density visit:
https://brainly.com/question/15164682
#SPJ1
Assume that 8.5 L of iodine gas (I2) are produced at STP according to the following balanced equation:
2KI (aq) + Cl2 (g) --> 2KCl (aq) + I2 (g)
a. How many moles of I2 are produced? ________ moles I2 (3 sig figs)
b. How many moles of KI were used? _________ moles KI (3 sig figs)
c. How many grams of KI were used? _________ grams KI (3 sig figs)
50 points
Answers
The molar volume of any gas behaving ideally at STP occupies a volume of 22.414 L. The number of moles of I₂ produced is 0.379 , number of moles of KI used is 0.758 , the grams of KI used is 126 g.
What is mole?One mole of a substance is defined as that quantity of it which contains as many entities as there are atoms exactly in 12 g of carbon - 12. The formula used to calculate the number of moles is:
Number of moles (n) = Given mass / Molar mass
Here at STP V = 8.5 L, P = 1 atm, T = 273 K. Then the value of 'n' is calculated from the ideal gas equation :
PV = nRT
1 × 8.5 = n × 0.0821 × 273
n = 8.5 / 0.0821 × 273
n = 0.379 mole
So number of moles of I₂ produced is 0.379 mole.
In the given equation, 2KI (aq) + Cl₂ (g) --> 2KCl (aq) + I₂ (g)
2 mole of Kl reacts with 1 mole of Cl₂ to give 2 mole of KCl and 1 mole of I₂ .
The number of moles of KI used = 0.379 × 2 = 0.758 mole
The mass of KI used = n / Molar mass of KI = 0.758 × 166.0 = 125.82 g ≈ 126 g
Thus,
a. 0.379 moles I₂
b. 0.758 moles KI
c. 126 g KI
To know more about moles, visit;
https://brainly.com/question/19730733
#SPJ1
If you burn yourself in lab you should?
A. See the nurse after class
B. Tell the instructor
C. See a doctor after school
D. Apply first aid yourself
Answers
Answer:
B. Tell the instructor
Explanation:
Always to the instructor about any accidents happens in a lab.
Answer:
B
Explanation:
Just helping out:)
Predict the products in the chemical reaction, Na+AlN
Answers
What is the binding energy for the nuclide 199F (atomic mass: 18.9984 amu) in MeV per nucleus?
Answers
The binding energy per nucleon for the ¹⁹F nucleon is equal to 7.786 MeV/nucleon.
What is binding energy?Binding energy can be defined as the minimum quantity of energy that is required to remove the particle from the system. Nuclear binding energy can be described as the energy required to dismantle a nucleus of an atom into free neutrons and protons.
The binding energy will be determined from the mass defect. Mass defect is calculated from the difference between the mass observed and the expected combined mass.
Given the mass of the ¹⁹F = 18.9984 a.m.u.
The mass defect for the ¹⁹F can be calculated as:
Δm = \((M _n +M_p) - M_F\)
\(\triangle m =( 9\times 1.0078 + 10 \times 1.0087 )- 18.9984\)
\(\triangle m =0.1588 \;a.m.u.\)
The binding energy for the fluorine can be calculated as:
E = Δmc²
E = 0.1588 × 931.5
E = 147.92 MeV
The binding energy per nucleon of ¹⁹F can be calculated as:
B.E.N. = 147.92/18.9984 = 7.786 MeV per nucleon
Learn more about binding energy, here:
https://brainly.com/question/10095561
#SPJ1
A frog sitting at the edge of a water puddle. The mud and dirt surrounding the pond is labeled A, the frog is labeled B, and the water is labeled C.
Fill in the blank with the correct sphere label.
A:
geosphere
B:
biospere
C:
hydrosphere
Answers
Part A is labeled as geosphere, B is biospere and C is hydrosphere.
What are the different division of the earth?
The Earth can be divided into four interconnected spheres: the geosphere, atmosphere, hydrosphere, and biosphere.
The geosphere refers to the solid, rocky part of the Earth, including the crust, mantle, and core. The mud and dirt surrounding the water puddle would be considered part of the geosphere.
The biosphere includes all living things on Earth and the environments in which they live. The frog sitting at the edge of the water puddle is part of the biosphere.
The hydrosphere refers to all the water on Earth, including oceans, lakes, rivers, and groundwater. The water in the puddle would be part of the hydrosphere.
These spheres are interconnected and influence each other. For example, the biosphere relies on the geosphere for nutrients and minerals, while the geosphere is shaped by the movement of water in the hydrosphere.
Learn more about hydrosphere here: https://brainly.com/question/1699547
#SPJ1
How many hydrogen atoms are in 709 grams of water? Answer in units of atoms.
Answers
Answer:
26 Hydrogen atoms
Explanation:
H2O
Each hydrogen atom: 2+16 = 18g
Hence,
1 atom -> 18g
x atoms -> 709g
709/18 = 39 atoms
Therefore, 39 atoms give 709g
Hence, 26 Hydrogen atoms are used
Feel free to mark it as brainliest :D
i really need help with this
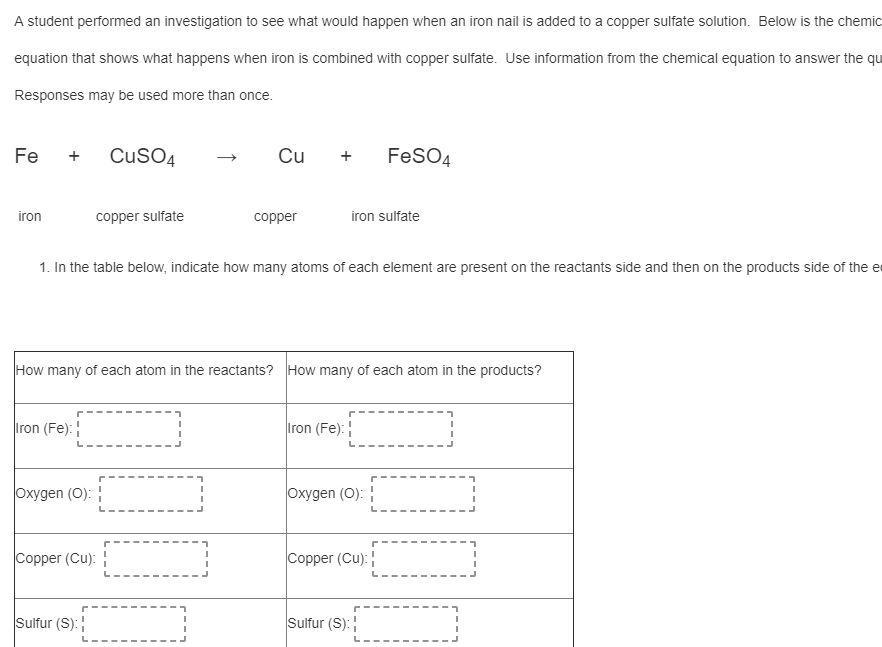
Answers
Answer:
i hope i can help you with this
Answer: focus in class
Explanation:
:////
Calculate the number of days after the explosion for a sample of iodine-131 to go from 100%
iodine-131 to less than 1% iodine-131. Complete chart.

Answers
It will take 56 days to go from 100% iodine-131 to less than 1% iodine-131.
From the table, we can see that the half life of iodine-131 is 8 days. We can see that it takes 8 days to have 50% of iodine-131
After 16 days, we will have 25 % of iodine-131
After 24 days, we will have 12.5 % of iodine-131
After 32 days, we will have 6.25% of iodine-131
After 40 days, we will have 3.125% of iodine-131
After 48 days, we will have 1.5625% of iodine-131
After 56 days, we will have 0.78125% of iodine-131
After 56 days, the percentage of iodine-131 drops to less than 1%.
Learn more: https://brainly.com/question/22824409
PLEASE HELP IMMEDIATELY I NEED THE ANSWER NOT A HINT THANK YOU
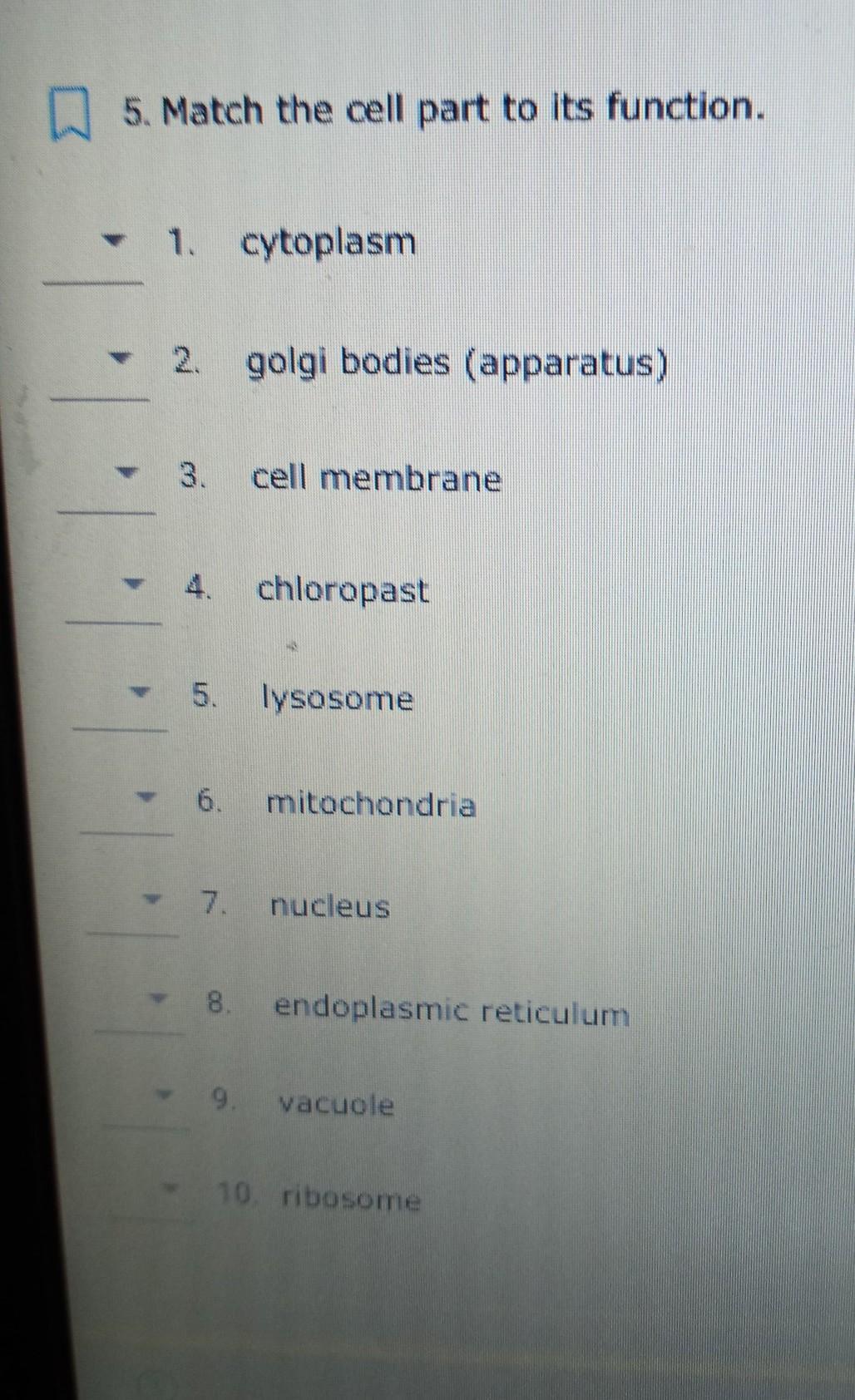
Answers
Cytoplasm: gel like environment which allows organelles to move about the cell
Golgi bodies: packages and ships materials out of the cell
Cell membrane: controls what goes in and out of the cell
Chloroplast: makes food for plant cells using sunlight
Lysosome: breaks down waste, food, and worn out cell parts
Mitochondria: breaks down food to release energy for the cell
Nucleus: contains the cell's DNA and is the control center of the cell
Endoplasmic reticulum: transports materials within cell; process lipids
Vacuole: stores water, waste and food
Ribosome: make proteins
write one common thing between condensation and hydrolysis
Answers
A chemistry graduate student is studying the rate of this reaction:
NH4OH(aq)→NH3(aq)+H2O(aq)
She fills a reaction vessel with and measures its concentration as the reaction proceeds:
Time (minutes) NH4OH
0 0.200M
1.0 0.0895M
2.0 0.577M
3.0 0.0426M
4.0 0.0337M
Use this data to answer the following questions.
a. Write the rate law for this reaction.
b. Calculate the value of the rate constant.
Answers
Answer:
Rate = k[NH₄OH]²
k = 6.17
Explanation:
We have concentrations of NH₄OH along with the given times. To determine the rate law of the reaction we need to determine first the order of reaction. This reaction can be order zero, first or second order. The expressions for each are the following:
Zero order:
k = [A₀] - [A] / t
First order:
k = 1/t * ln([A₀]/[A])
Second order:
k = (1/t) * (1/[A₀] - 1/[A])
And from here, the next part is easier. We just need to determine hat order is, calculating the value of k at two different times. If the value of k is constant, then we can say that the reaction is of that order.
Let's suppose its order zero (t = 1 and t = 2, [A₀] = 0.200 M):
k1 = 0.2 - 0.0895 / 1 = 0.1105
k2 = 0.2 - 0.577 / 2 = -0.1885
From this results we can conclude it's not zero order.
Let's suppose its order 1:
k1 = ln(0.2/0.0895) / 1 = 0.8041
k2 = ln(0.2/0.577) / 2 = 0.1733
It's not first order either, so we can conclude that this reaction is of 2nd order and the rate law would be:
Rate = k[NH₄OH]²Now that we know it's a second order reaction, we can determine the value of k using its expression:
k = (1/t) (1/[A] - 1/[A₀])
k = ln(1/0.0895 - 1/0.2) (1/1)
k = 6.17
And to confirm this value, let's calculate k for t = 2 s
k = (1/2) (1/0.0577 - 1/0.2)
k = 6.17The value is constant, so this is the true value of k.
Hope this helps
What is the density of Ar(g) at -11°C and 675 mmHg?
Answers
Answer:
The Density Of Ar (g) At -11°C And 675 MmHg (R =0.08206 L·atm/mol·K, 1 Atm = 760mmHg).
write the structural formula for 2-bromo-3-chloro-4,4-dimethylpentanal
Answers
Answer:
Br-CH2-CH(CH3)2-C(Cl)H-CH(CH3)2-CHO
Explanation:
The molecule has a total of 14 carbon atoms, 13 hydrogen atoms, and 1 bromine atom. The carbon atoms are arranged in a chain with a methyl group attached to the second carbon atom, a chlorine atom attached to the third carbon atom, and two methyl groups attached to the fourth carbon atom. The fifth carbon atom has a carbonyl group attached to it.
The molecule is an aldehyde, which means that it has a carbonyl group (C=O) at the end of the chain. The carbonyl group is polar, and the oxygen atom has a partial negative charge. The hydrogen atom has a partial positive charge. This polarity makes the aldehyde group susceptible to nucleophilic attack.
The bromine and chlorine atoms are both electrophilic, which means that they have a partial positive charge. This makes them susceptible to nucleophilic attack.
The methyl groups are non-polar and do not have any significant reactivity.
The molecule is a chiral molecule, which means that it has a mirror image that is not superimposable on itself. This is because the carbon atom with the carbonyl group is attached to four different groups.
The molecule is a liquid at room temperature and has a strong odor. It is used in a variety of products, including perfumes, flavorings, and plastics.
. What is that substance that yields hydrogen ion [H] when added with water?
Answers
2. What happens to the pH when you add more H+ ions to a solution that has no buffers?
Answers
Which enthalpy change can be calculated using bond enthalpies and/or average bond enthalpies only?
A. 2C3H6(g) + 9O2(g) → 6CO2(g) + 6H2O(l)
B. C5H10(l) + Cl2(g) → C5H10Cl2(l)
C. C5H10(l) + H2O(l) → C5H11OH(l)
D. C3H6(g) + H2(g) → C3H8(g)
Answers
The enthalpy change of the reaction C3H6(g) + H2(g) → C3H8(g) can be calculated based on bond enthalpies
What is enthalpy change?The term enthalpy change is defined as the heat evolved or absorbed in a reaction. The bond energies of reactants and products can be used to calculate the enthalpy change of the reaction when the substances are in the same phase.
As such, the enthalpy change for the reaction C3H6(g) + H2(g) → C3H8(g) can be calculated based on bond enthalpies and/or average bond enthalpies only.
Learn more about enthalpy change: https://brainly.com/question/1301642
2. A company makes mixtures of acetic acid and water such that the acetic acid is 15% of the total mass (weight) of the mixture. Let A be an unspecified number of grams of acetic acid, which can vary and let W be the corresponding number of grams of water in this type of mixture.
An equation that relates A and W is A = (3/17) W.
Answers
The equation that relates A and W, considering the desired 15% acetic acid concentration, is 3W = 2.55M.
The equation A = (3/17)W represents the relationship between the mass of acetic acid (A) and the mass of water (W) in the mixture. It states that the mass of acetic acid is equal to three seventeenths (3/17) of the mass of water.
Since the company wants the acetic acid to be 15% of the total mass of the mixture, we can set up another equation to represent this requirement. Let M be the total mass of the mixture. The mass of acetic acid (A) is 15% of the total mass, so we have A = 0.15M.
Now we can substitute A in terms of W from the first equation into the second equation: (3/17)W = 0.15M. We can simplify this equation by multiplying both sides by 17 to get 3W = 2.55M.
This equation allows the company to calculate the mass of water (W) required for a given mass of acetic acid (A) to maintain the desired concentration in the mixture.
For such more questions on concentration
https://brainly.com/question/26175405
#SPJ8
What type is hydropower?
Answers
Answer:
Hydroelectric energy, also called hydroelectricity or hydropower, is a form of energy that uses the power of flowing water to generate electricity. There are three main types of hydroelectric energy: impoundment, diversion, and pumped storage.
Explanation:
Which line for wood burning would be longer on the energy graph?
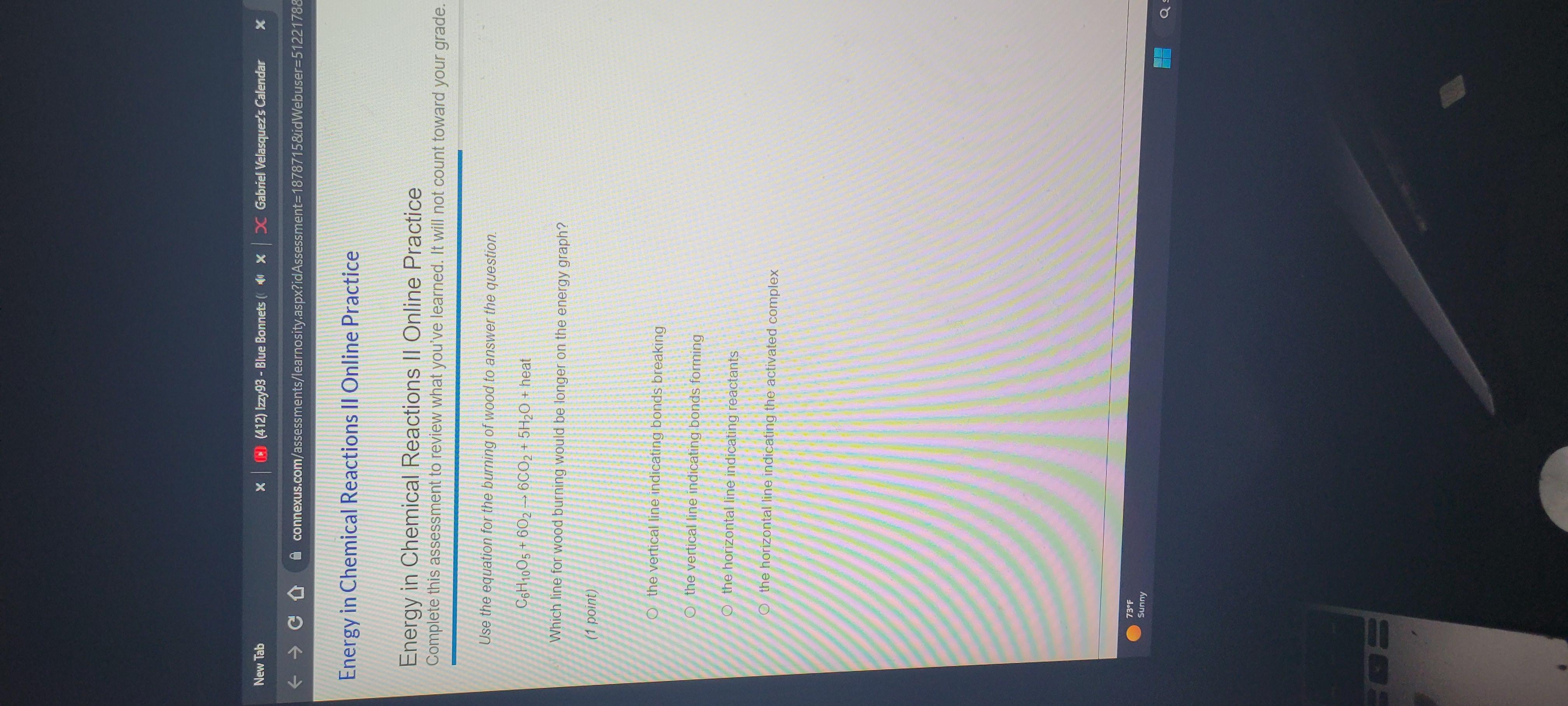
Answers
For the given reaction, the vertical line indicating bond breaking would be longer on the energy graph. Therefore, the correct option is option A.
What is combustion?combustion is a chemical process that often involves the presence of oxygen and produces light and heat with in form of flames. The chemical reaction's nature and the fact that more energy is produced than can be released into the environment.
Both contribute to the high rate as well as speed with which the reactants mix. For the given reaction, the vertical line indicating bond breaking would be longer on the energy graph.
Therefore, the correct option is option A.
To know more about combustion, here:
https://brainly.com/question/14335621
#SPJ1
Answer:
the vertical line indicating bonds forming
Explanation:
I took the test
Besides the major types of radioactive decay, there are two others: positron emission and electron capture.
1. Compare and contrast positrons with electrons.
2.Explain how positron emission works and how it causes transmutations.
3. Explain how electron capture works and how it causes transmutations.
4. Compare the transmutations caused by positron emissions and electron capture.
Answers
positron emission and electron capture both occur in specific radioactive decays and are associated with unstable nuclei. They play a crucial role in balancing the ratio of protons to neutrons in a nucleus, leading to more stable configurations.
Positrons and electrons are both subatomic particles with opposite charges. Positrons have a positive charge (+1), while electrons have a negative charge (-1). They have the same mass, which is approximately 9.1 x 10^-31 kilograms.
However, positrons and electrons differ in their origins. Positrons are the antiparticles of electrons, meaning they have the same mass but opposite charge. Positrons are typically produced in certain radioactive decays, while electrons are ubiquitous in atoms and play a fundamental role in chemical reactions.
Positron emission occurs when a proton inside an unstable nucleus is converted into a neutron, releasing a positron and a neutrino. This process reduces the atomic number by one while maintaining the mass number. The positron is ejected from the nucleus, carrying away the positive charge.
The positron can cause transmutations by colliding with an electron in the vicinity. The collision results in the annihilation of both particles, converting their masses into energy in the form of gamma rays. This annihilation process contributes to medical imaging techniques like PET scans.
Electron capture happens when an unstable nucleus captures an electron from its electron cloud. The captured electron combines with a proton in the nucleus, resulting in the formation of a neutron and a neutrino. This process also reduces the atomic number by one while preserving the mass number.
Electron capture causes transmutations by changing the composition of the nucleus. By capturing an electron, the number of protons decreases, transforming the element into another one with a lower atomic number.
Positron emissions and electron capture both result in the reduction of atomic number by one. However, positron emission involves the release of a positron from the nucleus, while electron capture involves the capture of an electron by the nucleus. The overall effect is the same—a decrease in atomic number.
Furthermore, positron emission and electron capture both occur in specific radioactive decays and are associated with unstable nuclei. They play a crucial role in balancing the ratio of protons to neutrons in a nucleus, leading to more stable configurations.
For more question on protons
https://brainly.com/question/1481324
#SPJ8
A sample of copper absorbs 4.31E+1 kJ of heat, resulting in a temperature rise of 6.71E+1 °C. Determine the mass (in kg) of the copper sample if the specific heat capacity of copper is 0.385 J/g°C.
Answers
Answer: 1.67 kg
Explanation:
The quantity of heat required to raise the temperature of a substance by one degree Celsius is called the specific heat capacity.
\(Q=m\times c\times \Delta T\)
Q = Heat absorbed=\(4.31\times 10^1kJ\) = \(43100J\) (1kJ=1000J)
m= mass of substance = ?
c = specific heat capacity = \(0.385J/g^0C\)
Change in temperature ,\(\Delta T=T_f-T_i=6.71\times 10^1^0C=67.1^0C\)
Putting in the values, we get:
\(43100J=m\times 0.385J/g^0C\times 67.1^0C\)
\(m=1670g=1.67kg\) (1kg=1000g)
Thus the mass (in kg) of the copper sample is 1.67
One mole of any gas occupies 22.4 L under certain conditions of temperature and pressure. Assume those conditions for this question.
In a spacecraft, this reaction occurs:
CO2(g)+2LiOH(s)→CaCO3(s)+H2O(l)
How many liters of carbon dioxide will 2 moles of lithium hydroxide (LiOH) absorb?
Answer choices: A .3.0 L B .6.0 L C. 23 L D 45 L
Answers
CO2(g) + 2LiOH(s) -> CaCO3(s) + H2O(l)
We can use this information along with the given molar volume of a gas at standard temperature and pressure to calculate the volume of carbon dioxide that will react with 2 moles of LiOH.
2 moles of LiOH will react with 1 mole of CO2, according to the stoichiometry of the balanced equation. Therefore, we need to find the volume occupied by 1 mole of CO2, which is 22.4 L at standard temperature and pressure.
So, the volume occupied by 1 mole of CO2 is 22.4 L.
Thus, the 2 moles of LiOH will require 1 mole of CO2 to react, which is equivalent to 22.4 L of CO2.
Therefore, 2 moles of LiOH will absorb 22.4 L of CO2.
Determine the net number of sigma bonds, the net number of pi bonds, and the overall bond order for N2+. Use 0.5 to indicate a fractional bond order.
Answers
Answer:
Net number of sigma bonds = 1
Net number of pi bonds = 2
Overall bond order = 3
Explanation:
Electronic configuration of N2 ia
1s2 2s2 2p3
There is head to head overlap in pz orbital. Thus, there is one sigma bond
Pi bond is formed whenever there is side wise overlapping. Since both px and py undergoes overlapping to form pi bond, there are two pi bonds
Bond order = 0.5 (bonding electron – antibonding electron)
= 0.5 (8-2) = 0.5*6 = 3
Answer:
Use 0.5 to indicate a fractional bond order.
σ bonds = 0.5
π bonds = 2
overall bond order = 2.5
Explanation:
trust me bro