1. Nitrogen and hydrogen gases are mixed in a closed 3500 mL flask. They react to form ammonia gas. At equilibrium, the mixture contains 0.25 mol of ammonia gas and 0.080 mol hydrogen gas. The equilibrium constant for this reaction is 5.81 x 10^5. What amount of nitrogen gas is present at equilibrium? All the entities are in gaseous form.
Answers
The amount of nitrogen gas present at equilibrium is 0.0039 moles.
Volume of the system = 3500 mL or 3.5 L
Concentration of ammonia at equilibrium = 0.25 mol/3.5 L = 0.07 M
Concentration of hydrogen gas at equilibrium = 0.080 mol//3.5 L = 0.02 M
Equilibrium constant for this reaction = 5.81 x 10^5
The reaction for the formation of ammonia is;
N2 + 3H2 ⇄ 2NH3
So;
K = [NH3]^2/[N2] [H2]^3
K [N2] [H2]^3 = [NH3]^2
[N2] = [NH3]^2/K [H2]^3
[N2] = [ 0.07]^2/ 5.81 x 10^5 × [0.02]^3
[N2] = 0.0011 M
Amount of N2 = 0.0011 M × 3.5 L = 0.0039 moles
Learn more: https://brainly.com/question/17960050
Related Questions
draw a potentail energy diagram for a combustion reaction
Answers
Potential energy diagram for a combustion reaction:. CLICK ON IMAGE.
In a typical combustion reaction, the reactants (e.g. a fuel and an oxidizer) are initially at a relatively high potential energy. As the reaction proceeds, the potential energy of the system decreases, and the products (e.g. carbon dioxide and water vapor) are at a lower potential energy than the reactants. The difference in potential energy between the reactants and products corresponds to the heat released during the reaction.
The diagram shows the initial energy level of the reactants, the activation energy required to initiate the reaction, and the final energy level of the products. The activation energy is the minimum amount of energy required for the reaction to occur.
Once the reactants have absorbed enough energy to reach the activation energy threshold, the reaction proceeds spontaneously and releases energy as it progresses to the lower-energy products.
Note that the shape of the potential energy diagram can vary depending on the specific reaction and the reaction conditions. For example, some reactions may have more complex energy profiles with multiple intermediate steps or energy barriers.
For more question on Potential energy click on
https://brainly.com/question/18963960
#SPJ11

Hurricanes and tropical storms gain their power from heated water evaporating from the ocean. Write a paragraph explaining why hurricanes typically weaken as they move into areas of cool water or over land.
Answers
As the disturbance gets to the land the warm ocean waters are no longer around thus the hurricane would die off rapidly
What is hurricane?The hurricane tends to occur when there is a rise of the warm air over the ocean. It should be noted that in the event of the hurricane there would be a sudden rising of the sea and this is going to rise so high that the violent waves would now begin to invade the land and cause a lot of destruction on its path as it is moving out from the ocean where the disturbance started.
Hence we can see that the origin of the hurricane is from some sort of a convection current that is taking place in the ocean and the hurricane must also be sustained by the existence of this convection current as the disturbance is moving away from the sea.
Learn more about Hurricane:https://brainly.com/question/13661501
#SPJ1
Describe the structure of fatty acids, triglycerides phospholipids, and steroids.
Answers
The structure of fatty acids involves long carbon-based chains with a carboxyl group (―COOH) located in the end, triglycerides phospholipids contain a phosphate group and glycerol, while steroids are cyclic and they contain C atoms linked via 4 fused rings.
What are lipids?Lipids are a broad category of biomolecules that include fatty acids, triglycerides phospholipids, and steroids. For example, fatty acids are long carbonated chains that contain exactly identical CH2 groups in the interior of the molecule and a carboxyl group (―COOH) located at one extreme side.
Triglycerides phospholipids are composed of three fatty acids chains linked to a glycerol backbone and also a phosphate group that may confer polar properties to the molecule.
Finally, steroids are another important class of lipids composed of several carbon-based fused rings.
Therefore, we can conclude that fatty acids, triglycerides phospholipids, and steroids are structurally different lipids that are mainly composed of carbon-based structures.
Learn more about the structure of lipids here:
https://brainly.com/question/1781782
#SPJ1
What is carbon black? How is it prepared,andwhat are some uses
Answers
Answer:
Carbon black is a form of paracrystalline carbon that has a high surface-area-to-volume ratio, albeit lower than that of activated carbon. it is prepared by methane Carbon black is mainly used to strengthen rubber in tires, but can also act as a pigment, UV stabilizer, and conductive or insulating agent in a variety of rubber, plastic, ink and coating applications.
Explanation:
plss mark me brainliest :) it would mean lot to me!!!!!
If you want there to be less of you tomorrow than today... you need to lose
Answers
Answer:
rise your words, not your voice rain grows flowers thunder does not.
Explanation:
off the top of my head
k12 What is the smallest particle representing oxygen
A. diatomic oxygen
B. a proton
C. a compound
D. an atom
Answers
Answer:
d
Explanation:
Answer:
An atom
Explanation:
I took test plz mark brainliest
(b) An unknown volume of 3.50 M potassium phosphate, K,PO, solution is added to 0.210 L of water
to form a 0.700 M K3PO, solution. Calculate the molarity of potassium ions, K in the solution.
Answers
Answer:
\(M_{K^+}=2.1M\)
Explanation:
Hello!
In this case, since we know the initial and final concentrations and the added volume of water, we can write:
\(3.50M*V_1=0.700M(0.210L+V_1)\)
Which can be solved for the initial volume as follows:
\(3.50M*V_1-0.700M(0.210L+V_1)=0\\\\3.50V_1-0.147-0.7V_1=0\\\\2.8V_1=0.147\\\\V_1=\frac{0.147}{2.8}=0.0525L\)
It means that the final volume is:
\(V_2=0.210L+0.0525L=0.2625L\)
Next, we compute the moles of potassium phosphate in solution:
\(n_{K_3PO_4}=0.2625L*0.700\frac{molK_3PO_4}{L}=0.184molK_3PO_4\)
Then, since 1 mol of potassium phosphate has 3 moles of potassium (K's subscript), we compute the moles of potassium ions:
\(n_{K^+}=0.184molK_3PO_4*\frac{3molK^+}{1molK_3PO_4}=0.551molK^+\)
Finally, the concentration of potassium ions turns out:
\(M_{K^+}=\frac{0.551molK^+ }{0.2625L}\\\\ M_{K^+}=2.1M\)
Regards!
20 PTS Which explains the differences in the distribution of solar energy on Earth's surface? (1 point)
A. Earth’s climate system determines how much energy is absorbed from the sun.
B. Earth’s gravitational force pulls the sun’s rays away from the equator.
C. Earth’s solar energy is dependent on air currents.
D. Earth’s curved shape and tilt on its axis do not allow the sun’s rays to fall evenly on its surface.
Answers
The differences in the distribution of solar energy on Earth's surface is due to Earth’s curved shape and tilt on its axis do not allow the sun’s rays to fall evenly on its surface.
The correct option is D.
What is solar energy?Solar energy refers to the energy produced by the Sun.
Solar energy is in the form of heat energy, light energy, and ultraviolet radiation.
The shape of the affects the amount of solar energy that reaches any region of the earth. The earth is spherical in shape. Hence, there is an uneven distribution of solar energy from the sun o the Earth. also, the tilt of the earth affects the amount of solar energy that reaches any region of the earth.
Learn more about solar energy at; https://brainly.com/question/27380261
#SPJ1
3. In the reaction of hydrogen with iodine_
A. iodine is oxidized
B. hydrogen is oxidized
C. hydrogen is reduced
Answers
Answer:
B. hydrogen is oxidized
Concept(s):
Hydrogen: A colorless, odorless, flammable gas that combines chemically with oxygen to form water: the lightest of the known elements. Symbol: H; atomic weight: 1.00797; atomic number: 1; density: 0.0899 g/l at 0°C and 760 mm pressure.
Iodine: A nonmetallic halogen element occurring at ordinary temperatures as a grayish-black crystalline solid that sublimes to a dense violet vapor when heater: used in medicine as an antiseptic. Symbol: l; atomic weight: 126.904; atomic number: 53; specific gravity: (solid) 4.93 at 20°C.
Oxidation: The deposit that forms on the surface of a metal as it oxidizes.
Reaction (Chemical/Physical):
⇒ Reaction, the reciprocal action of chemical gents upon each other; chemical change.
⇒ A change from one state (solid or liquid or gas) to another without a change in chemical composition which can be made by freezing, melting or boiling a substance. Chemical change.
More information:
This reaction is exothermic. Due to the attractive interactions between the ions, the reaction between positively charged hydride and negatively charged iodide releases heat energy. Additionally, the resulting hydrogen iodide is a more stable compound than the separate reactants.
Thank you,
Eddie
What might have been the advantages and disadvantages of just having
experienced polar explorers at the catlin arctic survey.
Answers
While having experienced polar explorers at the Catlin Arctic Survey would have been beneficial in many ways, it would also have been important for them to work collaboratively with the rest of the team.
Advantages:
Experienced polar explorers would have had a wealth of knowledge and skills, such as how to travel over the ice, how to set up camp, and how to handle emergencies.
Experienced polar explorers would have been able to make informed decisions about the best routes to take and the most efficient ways to travel. This could have helped to save time and energy.
Disadvantages:
Experienced polar explorers may have been set in their ways and resistant to new ideas. This could have hindered the team's ability to adapt to changing circumstances and make the most of new opportunities.
Experienced polar explorers may have been overconfident and taken risks that the rest of the team was not comfortable with. This could have put everyone's safety at risk.
To learn more about the Catlin Arctic Survey, follow the link:
https://brainly.com/question/28313250
#SPJ1
Chemistry, 50 points!!! Will also mark brainliest if you answer everything

Answers
Answer:
1. 2Al + Cl2 = Al2Cl2
2 TiCl4 + 2Na = Ti + 2NaCl2
3. H2O2 = H2O + O2
4. Na2S + 2HCl = H2S + 2NaCl
5. Mg(OH)2 + 2HCl = MgCl2 + 2H2O
1. 3O2 = 2O3
The pOH of a solution is 6.0. Which statement is correct?
Use pOH = -log[OH-] and PH+pOH = 14.
The pH of the solution is 20.0.
O The concentration of OH ions is 1.0 x 108 M.
The concentration of OH ions is 1.0 x 106 M.
O The pH of the solution is 8.0.
A
Answers
At pOH value of 6.0 the pH value of the following solution is 8.0 and the concentration of [\(H^{+}\) ] ion is \(10^{-8}\)
In this question we will apply the formula
pH +pOH = 14 . . . . . . . . . . . . .(1)
where pH = concentration of [\(H^{+}\) ] ion
pOH = concentration of [\(OH^{-}\) ] ion
As per the question
pOH =6.0
Putting the value of pOH in equation (1) we get the value of pH
pH + 6.0 =14
pH = 14 -6.0
pH = 8.0
The value of pH if the pOH value is 6.0 is 8.0
To find the concentration of \(H^{+}\) ion we will use the following formula
This is calculated by the formula
[\(H^{+}\)} = \(10^{-pH}\)
where we will write the values of pH
Hence the concentration of [\(H^{+}\)} ion is \(10^{-8}\)
Therefore at pOH of 6.0 the pH value of the following solution is 8.0 and the concentration of [\(H^{+}\) ] ion is \(10^{-8}\)
Read more about pH
https://brainly.com/question/11300720
The complete question is -
What is the pH value and concentration of [\(H^{+}\) ] ion of the following if the pOH value of the solution is 6.0 ?
_Na2S + _HCl -> NaCl + H2S
which of the following coefficients would balance this equation?
a. 2, 1, 2, 1
b. 1, 2, 1, 2
c. 1, 2, 2, 1
d. 2, 1, 1, 2
Answers
Answer:
C: 1, 2, 2, 1
Explanation:
_Na2S + _HCl -> NaCl + H2S (unbalanced)
1Na2S + 2HCl -> 2NaCl + 1H2S
Na2S + 2HCl → 2NaCl + H2S (Balanced)
Hope this helps:)
Answer:
c. 1,2,2,1
Explanation:
Na2S + 2HCl -> 2NaCl + H2S
someone please help!!
2 C4H10 + 13 O2 --> 8 CO2 + 10 H2O
Starting with 83.42 grams of Oxygen gas in excess C4H10, how much Carbon Dioxide can be created?
Box 1 = number
Box 2 = units
Box 3 = substance

Answers
Answer:
BOX 2
Explanation:
When 2 moles of NH3(g) react with N2O(g) to form N2(g) and H2O(g) according to the following equation, 880 kJ of energy are evolved. 2NH3(g) 3N2O(g)4N2(g) 3H2O(g) Is this reaction endothermic or exothermic
Answers
Answer:
Explanation:
This is a bit of a trick question.
Usually an exothermic reaction is written as
A + B - heat = C + D
The meaning of this equation is that when the bonds of the reactants break, heat has to be given away to the environment. On the left, exothermic means that heat has to be given.
The wording on this question means that heat is a product
A + B = C + D + heat.
In other words heat is given up to the environment. So this reaction is exothermic.
Used when your body moves. *
A. nuclear energy
B. radiant energy
C. chemical energy
Answers
Answer:
Hello! I think it is chemical energy. Nuclear energy can be ruled out and radiant energy is energy transfered using electromagnetic radiation. I hope this helped. Have a great day!
How do cells prepare for sexual reproduction?
A. Punnett Square B. Meiosis C. Mendel D. Trait
Answers
Answer:
Meiosis
Explanation:
They just split in four.
how much energy is required to heat 500g of ice at 0⁰C to 60⁰C?
a) 125,400 J
b) 167,000 J
c) 292,400 J
d) 41,883,600 J
Answers
The amount of energy needed to heat 500 g of ice at 0⁰C to 60⁰C is 292,400 J. Option C.
Energy of reactionIn order to calculate the energy required to heat the ice, we need to consider two stages: first, we need to calculate the energy required to melt the ice, and second, we need to calculate the energy required to heat the resulting liquid water to 60°C.
To melt the ice, we need to supply energy equal to the heat of fusion of ice. The heat of fusion of ice is 334 J/g. Therefore, the energy required to melt 500 g of ice is:
Q1 = (334 J/g) x (500 g) = 167,000 J
Once the ice is melted, we need to heat the resulting liquid water to 60°C. The specific heat capacity of water is 4.184 J/(g°C). Therefore, the energy required to heat 500 g of water from 0°C to 60°C is:
Q2 = (4.184 J/(g°C)) x (500 g) x (60°C - 0°C) = 125,520 J
The total energy required to melt the ice and heat the resulting liquid water to 60°C is the sum of Q1 and Q2:
Q = Q1 + Q2 = 167,000 J + 125,520 J = 292,520 J
Thus, the amount of energy needed to heat 500 g of ice at 0⁰C to 60⁰C is 292,400 J.
More on energy of reactions can be found here: https://brainly.com/question/1865119
#SPJ1
Daniella found an iron metal cube. The volume of the cube is 20 cm3 as shown below.
non
20.0 cm
If the density of iron is 7.9 g/cm3, what is the mass in grams of this iron cube?
A) 0.395 g
B) 2.53 g
C) 27.99
D) 158 g
Answers
Answer:b) 2.53
Explanation:
Identify the activated complex in the following reaction.
a. CuFeSO
b. FeFe
c. FeCuSO4
d. FeSO4
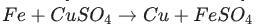
Answers
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. Option C)
An activated complex is a structure that exists temporarily during a chemical reaction and corresponds to the top of the energy barrier that must be overcome for the reaction to proceed to completion.
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. It is the structure with the greatest energy within the reaction process and is used to determine the rate at which the reaction occurs. An activated complex exists when the energy required to break the old bonds and form new ones has been absorbed. It has a specific configuration and energy content that is precisely defined.
A chemical reaction is the process by which atoms or groups of atoms in molecules interact to form new molecules. A chemical reaction is caused by the motion of electrons, which are negatively charged particles that surround atomic nuclei. The reaction proceeds through the formation of an intermediate species known as the transition state or activated complex. Reaction mechanisms are the sequence of steps involved in a chemical reaction. These steps describe the intermediate species formed as the reactants are converted to products. Hence option C) is correct.
for more questions on reaction
https://brainly.com/question/25769000
#SPJ8
A student measures the length of a book to be 32.4 cm. The accepted value of the length is 32.9 cm. What is the percent error of the student’s measurement?
Answers
The percent error of the student’s measurement is 98.48%
While doing any experiment or any calculation we usually calculate the estimated result or value that needs to come at the end of the reaction and that is called as theoretical value. But once the experiment is completed the value that we get is not the same as that of theoretical value and this value is called as the experimental value.
To understand the error that had happened during the experiment we find out the percent error and the formula for it is as follows
Percent error = Experimental value × 100
Theoretical value
When we use the same formula for the above given case we can see that the expected measurement was supposed to be 32.9 but the student measure it to be 32.4 so the percent error here is
= 32.4 × 100 ⇒ 98.48%
32.9
Hence the percent error in calculation is 98.48%
To know more about percent error
https://brainly.com/question/5463769
#SPJ1
COLOR THEME QQ ZOOM
13. Several JASON learning ambassadors for Aldine ISD recorded a
demonstration for the students.
They mixed two chemicals together and produced a 'golden rain'. The
equation for the reaction is:
KI + Pb(NO3)2
KNO3 + Pbl₂
1
The golden substance (image below) was found to be insoluble in water.
Which of the following compounds is the precipitate?
OKI
O
CLEAR ALL
Pb(NO3)2
KNO
Pbl₂
ADD NOTE
REFE

Answers
The compound that acts as the precipitate in this reaction is Pbl₂, or lead(II) iodide.
In the given reaction equation:
KI + Pb(NO3)2 → KNO3 + Pbl₂
The precipitate formed is Pbl₂, which is lead(II) iodide. In the reaction, potassium iodide (KI) reacts with lead(II) nitrate (Pb(NO3)2) to form potassium nitrate (KNO3) and lead(II) iodide (Pbl₂).
Lead(II) iodide is insoluble in water, as mentioned in the statement. When the two chemicals are mixed, lead(II) iodide is formed as a solid precipitate, which appears as a golden substance, as described.
For more questions on precipitate
https://brainly.com/question/17192312
#SPJ8
Please help, really need this turned in!
While working in the chemistry lab, you dissolve 2.5g of sodium hydroxide chips into a beaker containing 50mL of water. As you pick up the beaker to add it to a separate solution, you notice the outside of the beaker is very cold. What explains this decrease in temperature?
Question 45 options:
Energy was absorbed when the bonds between sodium and hydroxide ions were broken
Energy was gained when the sodium and hydroxide ions formed new bonds with the water.
Energy was released when the sodium and hydroxide ions came together to form NaOH.
Energy was released when the bonds between sodium and hydroxide ions were broken.
Answers
Answer:
Energy was gained when the sodium and hydroxide ions formed new bonds with the water.
Explanation:
The other answer choices are incorrect because:
Energy was absorbed when the bonds between sodium and hydroxide ions were broken: This is incorrect because breaking bonds requires energy, so energy is absorbed rather than released.
Energy was released when the sodium and hydroxide ions came together to form NaOH: This is incorrect because the reaction being described is dissolution of NaOH in water, not formation of NaOH from its constituent ions.
Energy was released when the bonds between sodium and hydroxide ions were broken: This is incorrect for the same reason as the first option. Breaking bonds requires energy, so energy is absorbed rather than released.
Answer: this correct answer will be energy was released when the sodium and hydroxide ions formed new bonds with the water.
Explanation:
Which of the following elements would be a good selection as the sacrificial electrode in the cathodic protection of tin (Sn)?
I. Silver
II. Copper
III. Nickel
IV. Magnesium
Answers
The elements that would be the good selection as the sacrificial electrode in the cathodic protection of tin, Sn is the correct option is III. Nickel and IV. Magnesium.
The Sacrificial anodes are helpful to protect the metal structures from the corroding. The Sacrificial anodes will work by the oxidizing to quickly as compared to the the metal that is protecting, and that is being consumed completely and before the other metal will reacts with the electrolytes. The Sacrificial anodes is the metals or the alloys that is attached to the hull that is more anodic.
Thus, the nickel and magnesium is used as the sacrificial electrode in the cathode protection of the tin.
To learn more about cathode here
https://brainly.com/question/13072489
#SPJ4
Changing a *blank* to a liquid to a gas is changing the phase of matter.
Answers
Answer:
solid
PLz give brain i need it to rank up
Explanation:
Definition
a visual representation of data that uses unconnected plotted points
Answers
4.08g of iron(II) chloride is dissolved in 50. mL of a 0.60 M aqueous solution of silver nitrate. Calculate the final molarity of chloride anion in the solution. you can assume the volume of the solution doesn't change when the iron(II) chloride is dissolved in it.
Answers
The final concentration of the chloride ions is 0.32 M.
What is the final molarity of the chloride ion?We know that we have to obtain the number of moles of the iron II chloride that was reacted in the solution and then we would have;
Number of moles of iron II chloride = 4.08g /127 g/mol = 0.032 moles
Given the fact that there are two chloride ions hence the amount of the chloride ions is 0.016 moles
The concentration of the silver nitrate = 50/1000 * 0.60 M
= 0.03 moles
Thus we would have a final concentration of 0.016 moles/ 0.05
= 0.32 M
Learn more about concentration:https://brainly.com/question/10725862
#SPJ1
What would be the mass of 4.640 x 10²1
particles of Aluminum (Al)?
Answers
The mass of 4.640 x \(10^{2}\) L particles of Aluminium is 33.67 g
The total number of molecules present in 22.4 litres of an element is equal to Avagadro's Number.
The value of Avagadro's number = \(6.023*10^{23}\).
To calculate the mass of \(4.640*10^{2}\) litres of Al, we first need to find the number of molecules present in it, then find the product of the number of molecules present will the mass of one molecule of Al atom.
Mass of Al=27
Number of molecules in 22.4 litres of Al = \(6.023*10^{23}\)
Number of molecules in 1 litre of Al = \(\frac{6.023*10^{23}}{22.4}\)
Number of molecules in \(4.640*10^{2}\) litres of Al= \(\frac{{6.023*10^{23}*4.640*10^{2}}}{22.4}\)
=\(1.247*10^{21}\)
Mass of \(1.247*10^{21}\) molecules of Al:
= \(1.247*10^{21}*27\) g
=33.669 g
=33.67 g
Read more about atomic mass from:
https://brainly.com/question/17067547
Read the given list of organisms.
rhinoceros, zebra, tortoise, rabbit, horse
What best describes the role of these five organisms in a food web? (3 points)
a
Carnivores, as they all obtain food from other animals
b
Herbivores, as they all obtain food from producers
c
Consumers, as they all eat either plants or meat
d
Consumers, as they all make their own food
Answers
Answer:
this would be c consumers as they all eat plants and/or meat
Explanation:
Answer:
(B) Herbivores.
Explanation:
Producers are plants and herbivores eat plants, and not meat, nor other animals, nor make their own food. I hope this helps good luck.
help asap 100pts -
What mass of H2 is needed to react with 8.75 g of O2 according to the following equation:
O2(g) + 4H2(g) → 2H2O(g)?
O 0.547 g H₂
O 2.21 g H₂
O 4.38 g H₂
O 17.5 g H₂
Answers
To solve this problem, we need to use stoichiometry and the balanced equation for the reaction. From the balanced equation, we see that 4 moles of H2 are required to react with 1 mole of O2 to produce 2 moles of H2O.
1 mole O2 = 32 g O2
8.75 g O2 = 8.75 g / 32 g/mol = 0.2734 moles O2
Using the stoichiometry of the balanced equation, we can determine the moles of H2 needed to react with the given moles of O2:
0.2734 moles O2 × 4 moles H2 / 1 mole O2 = 1.0936 moles H2
Finally, we can convert the moles of H2 to grams using the molar mass of H2:
1.0936 moles H2 × 2 g/mol H2 = 2.1872 g H2
Therefore, the mass of H2 needed to react with 8.75 g of O2 is approximately 2.19 g H2 (to two significant figures).
So, the closest option is O 2.21 g H₂.