Answers
Answer:
Not necessarily required, the water bath is used to give energy for reaction and it does not invlove in the reaction. So just normal water can be used in water bath.
Explanation:
Related Questions
what’s is the answer?

Answers
The energy of the photon of light can be obtained as 6.27 * 10^-20 J.
What is the energy of the photon?We know that a photon has to to do with a particular unit of light. We know that light can be said to be composed of very tiny corpuscles and these corpuscles of light is what we call the photon of the light.
We can be able to us the equation that is derived by Max Plank to be able to get the value of the energy of the photon of light. Now we know that a photon of light can have an energy that is able to be obtained by;
E = hf
h = Plank's constant
f = Frequency
Then;
E = 6.6 * 10^-34 Js * 9.5 * 10^13 Hz
= 6.27 * 10^-20 J
Thus as we can see from the parameters in the question, the energy of the photon is 6.27 * 10^-20 J.
Learn more about energy of the photon:https://brainly.com/question/2393994
#SPJ1
Which element would most likely have chemical properties similar to that of magnesium (Mg)? Ar Ca F Ni
Answers
Answer:
B. Ca is the answer
Explanation:
i took the test
The element that would most likely have chemical properties similar to that of magnesium (Mg) is calcium (Ca).
What is an element?Element is the simplest chemical substances that cannot be broken down in a chemical by chemical means and made up of atoms all having the same number of protons.
Elements in the same group in the periodic table are believed to have the same chemical properties and hence behave the same way chemically.
Magnesium and Calcium belong to the same group 2 of the periodic table, hence, would most likely have chemical properties that are similar.
Learn more about elements at: https://brainly.com/question/13025901
#SPJ9
the mass spectrum of an organic compound shows the relative abundances of m m to be 44.75% 44.75 % and m 1 m 1 to be 2.904%. 2.904 % . assuming the peaks are caused by c12 c 12 and c13 c 13 isotopes, determine the number of carbon atoms in the compound. the natural abundance of c12 c 12 is 98.93%, and the natural abundance of c13 c 13 is 1.07%. number of carbon atoms:
Answers
The compound contains approximately 0.1486.02210^23 carbon atoms, or about 8.9*10^22 carbon atoms. Thus, the number of carbon atoms in the compound is approximately 15.
Define molecular formula.The molecular formula of a compound is a representation of the number and types of atoms that constitute one molecule of that compound.
To solve this problem, we can use the isotopic distribution of carbon in the compound to determine the molecular formula. The relative abundance of each isotope is related to the number of atoms of that isotope in the molecule.
Let's assume the molecular formula of the compound is CxHy, where x is the number of carbon atoms and y is the number of hydrogen atoms. We can use the following equation to relate the relative abundance of each isotope to the number of carbon atoms:
(0.9893)x(0.4475) + (0.0107)x(0.02904) = 0.02904
Simplifying this equation, we get:
0.443x + 0.00031268x = 0.02904
0.44331268x = 0.02904
x = 0.06556/0.44331268
x = 0.148
Therefore, the compound contains approximately 0.1486.02210^23 carbon atoms, or about 8.9*10^22 carbon atoms. Thus, the number of carbon atoms in the compound is approximately 15.
Learn more about carbon atoms here:
https://brainly.com/question/13990654
#SPJ1
The number of carbon atoms in the compound can be determined by calculating the ratio of C12 to C13 isotopes present.
What is carbon atoms?Carbon atoms are the building blocks of life. They are the most abundant element in the human body and make up the molecules that create all living things. Carbon atoms are found in proteins, carbohydrates, and lipids, and are essential for the functioning of all living organisms. Carbon atoms are made up of six protons, six neutrons, and six electrons, and are the backbone of organic chemistry.
Since the relative abundances of C12 and C13 are 44.75% and 2.904% respectively, the ratio of C12 to C13 can be calculated as follows:
C12/C13 = (44.75/2.904) = 15.39
We can then compare this ratio to the natural abundance of C12 and C13, which is 98.93% and 1.07%, respectively.
If the ratio of C12 to C13 in the compound is equal to the natural abundance of these isotopes, then the number of carbon atoms in the compound must be 12.
C12/C13 = (98.93/1.07) = 92.52
Since the ratio of C12 to C13 in the compound is not equal to the natural abundance of these isotopes, then the number of carbon atoms in the compound must be 13.
To learn more about carbon atoms
https://brainly.com/question/27860158
#SPJ1
How are all atoms similar?
Answers
Answer:
Atoms are similar in the way that their nuclei contain only protons and neutrons.
Answer:
All things are made of atoms, and all atoms are made of the same three basic particles - protons, neutrons, and electrons. But, all atoms are not the same. The difference in the number of protons and neutrons in atoms account for many of the different properties of elements.
I hope this helps :)
the molecules of a solid normally move to and fro a little. When the solid is heated, its molecules start moving faster. The more it is heated, the faster they move. Can this be related in any way to change of state of the solid.How?
Answers
Define matrer?
a)Electrical conductivity
b)Anything that takes Iness and space
c) Something that doesn't take up space.
Answers
Answer:
if you are asking matter then
Explanation:
Matter is defined as anything that has mass and takes up space (it has volume).
A balloon is filled with 500.0 mL of helium at a temperature of 27 degrees Celsius and 755 mmHg. What volume in milliliters will it have when it reaches an altitude where the temperature is -33 degree Celsius and the pressure is 0.65 atm?
Answers
A balloon is filled with 500.0 mL of helium at a temperature of 27 degrees Celsius and 755 mmHg. 3.4 liters is the volume in milliliters will it have when it reaches an altitude where the temperature is -33 degree Celsius and the pressure is 0.65 atm.
A measurement of three-dimensional space is volume. It is frequently expressed in numerical form using SI-derived units or different imperial or US-standard units (such the gallon, quart, and cubic inch). Volume and length (cubed) have a symbiotic relationship.
The volume much a container is typically thought of as its capacity, not as the amount of space it takes up. In other words, the volume is the volume of fluid (liquid or gas) that the container may hold.
PV/T = constant
(755 x 500) / (27 + 273) = (494 x V) / (-33 + 273)
V = 3396 ml = 3.4 liters
To know more about volume, here:
https://brainly.com/question/1578538
#SPJ1
Which treatment(s) will help remove contaminants from minerals or from the pipes carrying water from a source? you can select more than one (Water Contamination Gizmos) **ONLY ANSWER IF YOU ACTUALLY KNOW ❗️❗️**
answer choices:
Sedimentation
Disinfection
Filtration
Coagulation

Answers
Sedimentation, filtration, and coagulation are the treatments that will help remove contaminants from minerals or from the pipes carrying water from a source.
Sedimentation is a process in which suspended particles settle out of water. It is one of the most basic techniques for removing particles from water. As particles settle, they become trapped in the bottom of a container or settle to the ground in an outdoor setting
Filtration is a method of removing particles from a fluid. It is a physical or chemical separation method that separates solids from fluids (liquids or gases) by adding a medium through which only the fluid can pass.
Coagulation is the process of using chemicals to remove contaminants from water. By creating a chemical reaction, coagulation destabilizes particles and causes them to clump together. This helps to remove the contaminants from the water.
Disinfection is the process of eliminating or destroying pathogens that cause infection. Disinfection eliminates harmful microorganisms by destroying or inactivating them. The disinfectant is a chemical or physical agent that is used to destroy or inactivate harmful microorganisms.
Know more about Filtration here:
https://brainly.com/question/29756050
#SPJ8
what formula was used to find the answer
Answers
How many decigrams are equal to 1.35 milligrams? 135 dg 13.5 dg 0.0135 dg 0.135 dg
Answers
Answer:
0.0135 dg
Explanation:
Answer: 0.0135 dg
Explanation:
the maximum amount of copper sulfate that can be dissolved in 45.0g of water at 70C is 20.0g. what is the solubility of copper sulfate at that temperature?
(in grams per 100g water)
Answers
Answer:
That depends on the amount. Solubility is the maximum amount of salt dissolved in 100 g of water. So, if the amount is within limit, then yes. Solubility of anhydrous copper sulfate is 24.3 g/100 g water, so it is not bad. Put it in perspective, the solubility of NaCl is about 35 g/100 g water at room temperature. If the amount of copper sulfate is unknown, qualitative I'd say solid copper sulfate will dissolve in water completely.
Explanation:
If you use the pentahydrate CuSO4.5H2O then the solubility at 20°C is 20.8g/100ml H2O
If you use the anhydride, CuSO4. then the solubility at 20°C is 36.2g/100ml H2O
4. Determine how many molecules are in 7.00 mol Fe2O3
Answers
I would divided it to get the anwser and then moloeces
Explanation:
Divide
You need to calculate the enthalpy change (AH) of the reaction A + 2B → C.
How can the enthalpies given for the reaction steps below be combined to
give the overall change in enthalpy?
D+B
D+3B
A. AH = AH2-A H₁
B. AH=2AH₁+AH²₂
OC. AH AH ₁ +AH²₂
D. AH = A H₁-AH₂
A
C
A
AH°,
A Hº
Answers
The enthalpy change of the reaction: A + 2B → C is determined as follows: ΔH = ΔH₁ + ΔH₂; option C
What is enthalpy change?Enthalpy change refers to the change in heat content as reactant molecules combine to form products.
The enthalpy change of a multistep reaction is calculated from by summing the enthalpy changes of the intermediate steps that leads to the overall reaction.
Thus, the enthalpy change of the reaction: A + 2B → C is determined as follows:
ΔH = ΔH₁ + ΔH₂
In conclusion, the enthalpy change of the reaction is determined from the summation of the enthalpy changes that occur in the intermediate steps.
Learn more about enthalpy change at: https://brainly.com/question/14291557
#SPJ1
what is the gulf stream.
a surface current
a deep current
a river
a climate event
Answers
Part A
A solution of cough syrup contains 5.00% active ingredient by volume. If the total volume of the bottle is 68.0 mL, how many milliliters of ac
ingredient are in the bottle?
Express your answer with the appropriate units.
Answers
There are 3.4 milliliters of the active ingredient in the cough syrup bottle.
To calculate the volume of the active ingredient in the cough syrup bottle, we need to multiply the total volume of the bottle by the percentage of the active ingredient.
Given:
Total volume of the bottle = 68.0 mL
Percentage of active ingredient = 5.00%
First, we convert the percentage to a decimal by dividing it by 100:
Percentage of active ingredient = 5.00% = 5.00/100 = 0.05
Next, we calculate the volume of the active ingredient:Volume of active ingredient = Total volume of the bottle × Percentage of active ingredient
Volume of active ingredient = 68.0 mL × 0.05
Volume of active ingredient = 3.4 mL
Therefore, there are 3.4 milliliters of the active ingredient in the cough syrup bottle.
It's important to note that the calculation assumes a homogeneous distribution of the active ingredient throughout the solution.
For more such questions on cough syrup visit:
https://brainly.com/question/2970281
#SPJ8
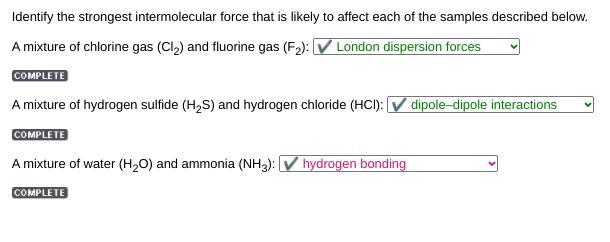
Identify the strongest intermolecular force that is likely to affect each of the samples described below.
A mixture of chlorine gas (Cl) and fluorine gas (F): V London dispersion forces
COMPLETE
Tweaks
Menu
A mixture of hydrogen sulfide (H2S) and hydrogen chloride (HCI): V dipole-dipole interactions
Search
Selection
COMPLETE
Guess
this
hydrogen bonding
A mixture of water (H2O) and ammonia (NH3):
Answers
Answer:
A mixture of chlorine gas (Cl2) and fluorine gas (F2):
✔ London dispersion forces
Explanation:
Warm air ___________________ and cold air __________________.
PLZ ILL GIVE BRAINLIEST!!!
Answers
Answer:
warm air rises and cold air decreases
Explanation:
cold: The absorbed energy moves and expands the molecules in the air, thus reducing the air density
hot: Hot air increases because it expands when you heat air.
Answer:
warm air rises and cold air decreases
Explanation:
cold: The absorbed energy moves and expands the molecules in the air, thus reducing the air density
hot: Hot air increases because it expands when you heat air.
Please help me!!!:)))

Answers
Answer:
blocks 1 and 2 the rhdh huff hgfhh5
list the 3 pKa's for H3PO4
Answers
Answer:
The three pKa values for phosphoric acid (H3PO4) are 2.12, 7.21, and 12.32.
2 dmcube of N2 at a pressure 100kpa and 5dmcube of H2 at pressure of 500kpa are injected into a 10dmcube container, calculate partial pressures of H2 and N2
Answers
The partial pressure of \(N_2\)is 165.6 kPa and the partial pressure of \(H_2\)is 434.4 kPa.
To calculate the partial pressures of \(H_2\)and \(N_2\)in the 10dmcube container, we need to use the ideal gas law equation, which states that the pressure of a gas is directly proportional to its number of moles and temperature, and inversely proportional to its volume.
First, we need to calculate the number of moles for each gas. Since we are given the volume of each gas and the volume of the container, we can use the formula:
Number of moles = Volume / Molar volume
The molar volume is the volume occupied by one mole of a gas at a given temperature and pressure. At standard temperature and pressure (STP), the molar volume is 22.4 L/mol.
For \(N_2\), the number of moles is 2 dmcube / 22.4 L/mol = 0.089 mol
For \(H_2\), the number of moles is 5 dmcube / 22.4 L/mol = 0.223 mol
Next, we can calculate the partial pressures of each gas using the formula:
Partial pressure = (Number of moles / Total number of moles) * Total pressure
The total pressure is the sum of the pressures of each gas:
Total pressure = Pressure of N2 + Pressure of \(H_2\)
Given that the pressure of N2 is 100 kPa and the pressure of \(H_2\)is 500 kPa, we have:
Total pressure = 100 kPa + 500 kPa = 600 kPa
Now we can calculate the partial pressure of \(N_2\):
Partial pressure of \(N_2\)= (0.089 mol / (0.089 mol + 0.223 mol)) * 600 kPa = 165.6 kPa
Similarly, we can calculate the partial pressure of \(H_2\):
Partial pressure of H2 = (0.223 mol / (0.089 mol + 0.223 mol)) * 600 kPa = 434.4 kPa
Therefore, the partial pressure of \(N_2\)is 165.6 kPa and the partial pressure of \(H_2\)is 434.4 kPa.
For more such question on partial pressure visit:
https://brainly.com/question/19813237
#SPJ8
The noble-gas notation for tin (Sn) will contain the symbol
O [Ar].
O [Kr].
O [Xe].
O [Rn].
Answers
Please help me ASAP I’ll mark Brainly

Answers
Answer:
Batteries hold chemical energy
Explanation:
The battery acid in a battery leads to chemical energy.
Which of the following happens during a chemical change? Check all of the boxes that apply.
One atom or more changes into atoms of another element.
New substances with different properties are formed.
Solids, liquids, or gases may form.
Reaction mixtures always give off some heat.
Reaction mixtures always need to be heated.
00
Answers
The correct options that apply during a chemical change are:
A) One atom or more changes into atoms of another element.
B) New substances with different properties are formed.
C) Solids, liquids, or gases may form. Option A, B and C
During a chemical change, the arrangement of atoms in molecules is altered, resulting in the formation of new substances with different chemical properties. This is represented by option B. For example, when hydrogen gas (H₂) reacts with oxygen gas (O₂), a chemical change occurs, and water (H₂O) is formed. The properties of water, such as boiling point, density, and chemical reactivity, are distinct from those of its constituent elements.
Additionally, during a chemical change, atoms can rearrange to form molecules of different elements, as indicated in option A. For instance, during a nuclear reaction, such as radioactive decay, the nucleus of an atom can change, leading to the formation of atoms of different elements.
Option C is also correct. Depending on the specific reaction conditions, chemical changes can result in the formation of solids, liquids, or gases. For example, when a metal reacts with an acid, such as zinc with hydrochloric acid, a gas (hydrogen) is produced.
Options D and E are not universally applicable to all chemical changes. While some reactions may release heat energy (exothermic reactions), others may absorb heat energy (endothermic reactions). The requirement for heating or the release of heat depends on the specific reaction and its energy considerations.
In summary, during a chemical change, atoms can change into atoms of another element (A), new substances with different properties are formed (B), and solids, liquids, or gases may form (C).
For more such questions on chemical change visit:
https://brainly.com/question/1222323
#SPJ8
There are eight diastereomers of 1,2,3,4,5,6-hexachlorocyclohexane. One of them is drawn. Draw the other seven in the cyclohexane framework below (5 points). One isomer loses HCl in an E2 reaction nearly 1000 times more slowly than the others. Circle the isomer that reacts so slowly, draw its corresponding chair conformers and explain the reason for the slow rate. (5 points).
Answers
Answer:
Attached below diagram of the eight diastereomers
The Isomer that reacts so slowly is DIASTEREOMER 8
Explanation:
The Isomer that reacts so slowly is DIASTEREOMER 8 in an E2 reaction and this is because no pair of chlorine and hydrogen atoms can assume the anti-periplanar orientation that is preferred in an E2 elimination
attached below is Diagram of the eight diastereomers ( screen shot from my drawing tool )


Propane boils at -42C. Octane boils at 126C Which is a gas at the freezing point of water?
Answers
The substance which is a gas at the melting point of water is the gas propane.
What is freezing point?The term freezing point refers to the point at which a liquid turns to solid. It is often the temperature as the melting point.
Thus, the substance which is a gas at the melting point of water is the gas propane.
Learn more about freezing point:https://brainly.com/question/3121416
#SPJ1
Which chemical equation is balanced?
O A. Fe + O₂ → Fe2O3
O B. 2 Fe + 0₂ - > Fe₂O3
O C. 2 Fe + 3 0₂ Fe2O3
D. 4 Fe + 3 0₂ 2 Fe2O3
Answers
Answer:
F
Explanation:
The balanced chemical equation is option D:
4 Fe + 3 O₂ → 2 Fe₂O₃
This is a balanced equation because:
- There are four iron (Fe) atoms on both the reactant and product sides.
- There are three oxygen (O₂) molecules on both the reactant and product sides.
- The coefficients are the smallest possible integers that make the equation balanced.
How many atoms are in 1.50 mol of carbon-12? How many grams does this much carbon-12 weigh
Answers
The number of atoms that are in 1.50 mol of carbon-12 is 18g.
1 mol of any substance contains 6.022×1023 atoms/molecules
So 1.50 moles of carbon will have 1.50×6.022×1023 atom = 9.0×1023 atoms
Now, number of moles = weight g /molar mass gmol-1
1.5= weight g/ 12 gmol-1.
So weight = 1.5 mol×12gmol-1 = 18 g
Mole is the unit for the measurement of quantity of a substance.The number of moles is used a unit of measure large number of atoms and the other components.Mole is the standard unit for amount of substance.The elementary numbers of a certain substance are present in a substance or sample is determined by the quantity of that material.To learn more about moles visit:
brainly.com/question/26416088
#SPJ1
How do ice and water on the ground affect incoming solar radiation? O They filter 22 percent of solar radiation that reaches the surface. They reflect 4 percent of solar radiation that reaches the surface. They absorb 100 percent of solar radiation that reaches the surface. O They scatter 5 percent of solar radiation that reaches the surface.
Answers
Answer:
They reflect 4 percent of solar radiation that reaches the surface.
Explanation:
When the sun angle is high and water is liquid notice, there is less reflection that takes place.
QUESTION 1
*Hint: Write this down on paper first, then try to enter your answers."
Write and balance the chemical reaction for the combustion of propane gas (C3H8). Every slot requires
an answer, think carefully about what should go where!
O
()
+
+
✪
1

Answers
The balanced chemical equation for the combustion of propane gas would be \(C_3H_8 + 5O_2 -- > 3CO_2 + 4H_2O\)
Balancing chemical equationsPropane gas burns in oxygen to yield carbon dioxide and water according to the following equation:
\(C_3H_8 + O_2 -- > CO_2 + H_2O\)
The equation above is not balanced because it does not conform to the law of conservation of atoms. According to this law, an equal number of atoms of different elements must be in the reactants and the products, irrespective of their forms.
Thus, the balanced version of the equation would be the following:
\(C_3H_8 + 5O_2 -- > 3CO_2 + 4H_2O\)
Thus, the slots can be filled accordingly to reflect the balanced equation of the reaction.
More on balancing chemical equations can be found here: https://brainly.com/question/28294176
#SPJ1
& Oregonians eat about 9503 metric tons of food each day. What is this consumption rate in grams
per second?
Answers
Answer:
109988 grams per second
Explanation:
To solve this problem first we convert 9503 metric tons into grams, keeping in mind that:
1 metric ton = 1000 kg1 kg = 1000 gMeaning that:
9503 metric ton * \(\frac{1000kg}{1metricTon}*\frac{1000g}{1kg}\) = 9503x10⁶ gThen we calculate how many seconds are there in one day:
1 day * \(\frac{24h}{1day} *\frac{60min}{1h} * \frac{60s}{1min}\) = 86400 sFinally we calculate the consumption rate:
9503x10⁶ g / 86400 s = 109988 g/s