Answers
Answer:
B
Explanation:
Answer:
B its correct because i did the test.
Explanation:
Related Questions
Cumulative Exam Active
41 42 43 144
The electron configuration of nitrogen (N) is
O 1s²2s²2p³
O 1s²2s²2p4
O 1s²2s²2p5
O 1s²2s²2p6
Answers
The answer is: The electronic configuration of Nitrogen is \(1s^22s^22p^3\).
Electronic configuration: The electronic configuration is defined as the distribution of electrons of an atom in the atomic or molecular orbitals and is written using the labels for the subshell.
How to decide which orbital is filled first?
The order in which electrons are filled in atomic orbitals as:(Shown in image)
Just follow the arrows to select the orbitals, s orbital can have 2 electrons, p can have 6 electrons, d can have 10 electrons and f can 14 electrons.The electronic configuration in which the outer shell is completely filled is known as noble-gas configuration as they are similar to electronic configurations of noble gases.Now, the given element is nitrogen (\(N\)). The atomic number of Nitrogen is 7. Thus, these 7 electrons are filled as-\(1s^22s^22p^3\)
Therefore, the electronic configuration of Nitrogen is \(1s^22s^22p^3\).To learn more about the electronic configuration, visit:
https://brainly.com/question/21977349
#SPJ4

Nitrogen's complete electron configuration is 12s2s22p3.
The shorthand electron configuration for noble gases is [He] 2s22p3. Nitrogen has an atomic number of 7. The nitrogen atoms' nucleus contain this many protons. An atom that is neutral has an equal number of protons and electrons. Thus, the ground state electron configuration will consist of 7 electrons in the suitable s and p orbitals (state of lowest energy). For nitrogen, the entire electron configuration is 1s22s22p. Scientists may easily express and explain how the electrons are organized around the nitrogen atom's nucleus by using the configuration notation for nitrogen (N). As a result, it is simpler to comprehend and forecast how atoms will cooperate to form chemical bonds.
Learn more about electronic configuration here-
https://brainly.com/question/11309892
#SPJ9
what is the number of each element and the total number of atoms for vinegar?

Answers
C =4
H=8
O=4
So the total atoms are 16
If heat transfers from the system (solute) to the surroundings (solvent), then AH is
negative (AH < 0), and the reaction is defined as * (T)
A)endothermic
B)exothermic
Answers
Answer:
B) exothermic.
Explanation:
Hello!
In this case, we need to keep in mind that exothermic reactions release heat, so they increase the temperature as the final energy is less than the initial energy; in contrast, endothermic reactions absorb heat, so they decrease the temperature as the final energy is greater than the initial energy.
In such a way, when a dissolution process shows off a negative enthalpy of dissolution, we infer it is an exothermic process due to the aforementioned; therefore, the answer is:
B) exothermic .
Best regards!
1. What do you understand about equivalent weight for lime requirement?
2. What is the relationship between normality and equivalence?
Answers
Answer:
Explanation:
What do you understand about equivalent weight for lime requirement?
If field data are not available, lime requirements can be determined with the solution is added to a given weight or volume of soil and the pH is measured. Each milligram per liter of sodium bicarbonate is equal to 0.56 mg/L of alkalinity. A winter wheat crop grown on a light sand soil in a low rainfall area will mean an
If you blew up a balloon inside an air-conditioned house and then brought it outside on a hot summer day, then what do you expect to happen to the balloon?
Answers
Answer:
You can expect the balloon to expand a little
Explanation:
The molecules in the balloon will be heated up once they feel the warmth of the summer heat and start moving a lot faster
1. Determine the reaction TYPE:2. Predict the product3. Use the names to write GOOD chemical formulas. (GOOD formulas have a NEUTRAL charge balanced using subscripts.)4. Balance the equation using coefficients C5H12 (burning) +_________________________________________
Answers
ANSWER
\(\begin{gathered} \text{ The balanced equation is } \\ \text{ C}_5H_{12(l)}\text{ }+\text{ 8CO}_{2(g)}\text{ }\rightarrow\text{ 5CO}_{2(g)}\text{ }+\text{ 6H}_2O_{(l)} \end{gathered}\)EXPLANATION
When an organic compound is burns in air, the type of reaction is called a combustion reaction.
The major products formed when an organic compound undergoes a combustion reaction are carbon dioxide and water.
To write a balanced equation for the reaction, follow the steps below
The combustion formula is written below as
\(\text{ C}_xH_y\text{ + x }+\frac{y}{4}O_2\rightarrow xCO_2\text{ + }\frac{y}{2}H_2O\)The compound given is pentane because it has 5 carbon atoms
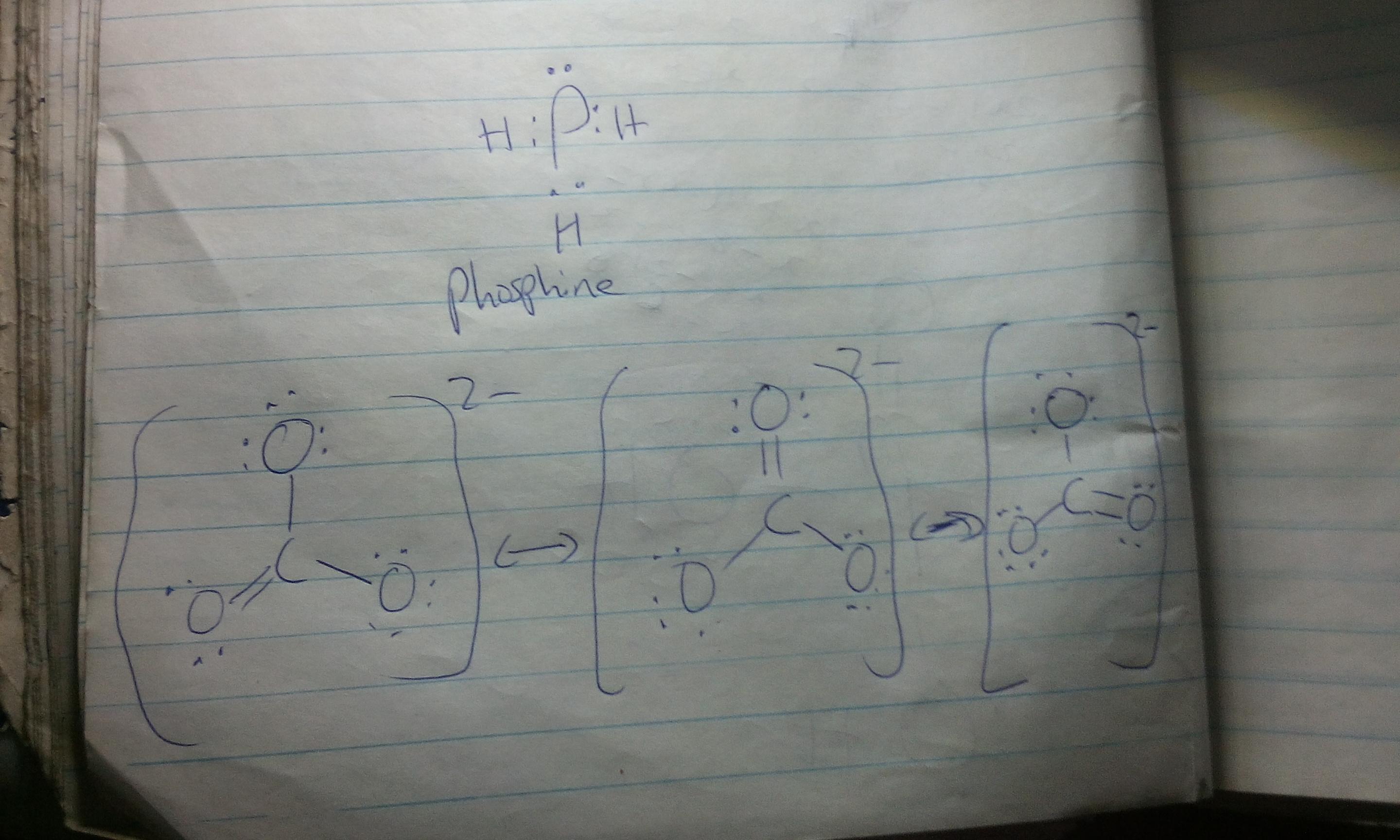
\(\begin{gathered} \text{ C}_5H_{12}\text{ }+\text{ 5}+\frac{12}{4}O_2\text{ }\rightarrow\text{ 5CO}_2\text{ }+\text{ 6H}_2O \\ \\ \text{ C}_5H_{12(l)}\text{ }+\text{ 8CO}_{2(g)\text{ }}\text{ }\rightarrow\text{ 5CO}_{2(g)}\text{ }+\text{ 6H}_2O(l) \end{gathered}\)Resonance Structures are ways to represent the bonding in a molecule or ion when a single Lewis structure fails to describe accurately the actual electronic structure. Equivalent resonance structures occur when there are identical patterns of bonding within the molecule or ion. The actual structure is a composite, or resonance hybrid, of the equivalent contributing structures. Draw Lewis structures for thecarbonate ion and for phosphine in which the central atom obeys the octet rule. ... How many equivalent Lewis structures are necessary to describe the bonding in CO32-
Answers
Answer:
See explanation
Explanation:
A Lewis structure is also called a dot electron structure. A Lewis structure represents all the valence electrons on atoms in a molecule as dots. Lewis structures can be used to represent molecules in which the central atom obeys the octet rule as well as molecules whose central atom does not obey the octet rule.
Sometimes, one Lewis structure does not suffice in explaining the observed properties of a given chemical specie. In this case, we evoke the idea that the actual structure of the chemical specie lies somewhere between a limited number of bonding extremes called resonance or canonical structures.
The canonical structure of the carbonate ion as well as the lewis structure of phosphine is shown in the image attached to this answer.
Write the pressure equilibrium constant expression for the reaction N2(g)+3H2(g)=2NH3(g)
Answers
Answer:
\(keq=\frac{[NH_{3} ]^2}{[N_{2} ][H_{2} ]^3}\)
Explanation:
A copper penny will sink in molten copper. What can you infer about the difference in distance between the molecules in a copper penny and in molten copper?
Answers
The molecules in a copper penny is closely packed and and has no space to move apart thus the material will be denser than that in the molten state. That's why the penny sink in the molten copper.
What is molten copper?Copper is a transition metal exhibiting all the metallic properties. The molten state of metals is the fluid state where the molecule are not strongly held by the metallic bonds.
Molten material is made by melting them and the liquid like state contains molecules with some space to move apart. Whereas, in solid state as in a copper penny, the molecules are closely packed and have no space to move apart.
An object will sink in a liquid if it is less dense than the liquid. Copper penny is denser than the molten copper because the molecules are densely packed and it will sink on to it.
To find more on density, refer here:
https://brainly.com/question/15164682
#SPJ1
3.Calculate the pH of a 0.250 M solution of potassium phenolate, KC6H5O. Ka for phenol (C6H5OH) is 1.0 x 10-10.
Answers
From the statement and the information available in the question, the pH of the solution is 12.2.
What is Ka?The term Ka refers to the acid dissociation constant of a substance, we can obtain this by setting up the ICE table as shown;
C6H5O^- + H2O ⇄ C6H5OH + OH^-
I 0.250 0 0
C -X +X +X
E 0.250 -x x x
Now;
Kb = 1 * 10^-14/ 1.0 x 10-10
Kb = 1 * 10^-4
Kb = [C6H5OH] [OH^-]/[C6H5O^-]
1 * 10^-4 = x^2/ 0.250 -x
1 * 10^-4 (0.250 -x) = x^2
2.5 * 10^-4 - 1 * 10^-4 x = x^2
x^2 + 1 * 10^-4 x - 2.5 * 10^-4 = 0
x=0.016 M
Given that; [C6H5OH] = [OH^-] =x
pOH = -log(0.016 M) = 1.8
pH = 14 - 1.8 = 12.2
Learn more about Ka: https://brainly.com/question/16236454
Calculate the volume of 0.5 M HCOOH and 0.5 M HCOONa required to prepare 0.1 L of pH 4.50 buffer with a buffer strength of 0.1 M. The pa of HCOOH is 3.75.
Answers
The volume of 0.5 M HCOOH required is 50 mL and the volume of 0.5 M HCOONa required is 150 mL.
To prepare a pH 4.50 buffer with a buffer strength of 0.1 M, we need to use a mixture of HCOOH and HCOONa in a specific ratio. The buffer equation for this system is:
\(pH = pKa + log([HCOO-]/[HCOOH])\)
Substituting the given pH of 4.50 and pKa of 3.75:
We can then use the Henderson-Hasselbalch equation:
\([HCOOH]/[HCOO-] = 3.16 \\\\[HCOOH] + [HCOO-] = 0.1 M\)
Solving these equations simultaneously, we get:
\([HCOOH] = 0.0253 M\) and \([HCOO-] = 0.0747 M.\)
Therefore, the volume required is 50 mL and the volume of 0.5 M HCOONa required is 150 mL, to prepare 0.1 L of pH 4.50 buffer with a buffer strength of 0.1 M.
To know more about Henderson-Hasselbalch equation, here
brainly.com/question/30466186
#SPJ1
1pt The step by step method scientists use to solve problems and test ideas is
O A. The Scientific Method
B. The Report
c. The Research
O D. Learning Objectives
Answers
The force of a hammer drives a nail into wood. This is an example of? A. An unbalanced force. B. Gravitational force. C. Friction. D. Balanced forces.
Answers
Answer:
The answer is A. an unbalanced force
Explanation:
This is because the force from the hammer on the nail causes a force that unbalanced normal forces on the nail.
Advantage of rusting
Answers
1. What are the two properties a force have that make
it a vector quantity? (Identify the force )
Answers
Answer:
magnitude and direction
I need help ASAP please giving brainliest!!

Answers
Explanation:
im bumb like really bumb
The correct value of m, n, x and y to obtain a balanced equation is?
m B2O3(s) + n HF(l) → x BF3(g) + y H2O(l)
a.
m=1, n=1, x=1 and y=1
b.
m=1, n=6, x=2 and y=3
c.
m=1, n=1.5, x=1 and y=1
d.
m=2, n=12, x=4 and y=6
Answers
Answer:
d
Explanation:
answer d makes the equation balance
b. How many moles of phosphorous pentachloride will react with 7.36 g of water?
PCl5+4H20–>5HCl+H3PO4
Answers
Answer:
0.102 mol
Explanation:
Step 1: Write the balanced equation
PCl₅ + 4 H₂O ⇒ 5 HCl + H₃PO₄
Step 2: Calculate the moles corresponding to 7.36 g of H₂O
The molar mass of H₂O is 18.02 g/mol.
7.36 g × 1 mol/18.02 g = 0.408 mol
Step 3: Calculate the moles of PCl₅ required to react with 0.408 moles of H₂O
The molar ratio of PCl₅ to H₂O is 1:4.
0.408 mol H₂O × 1 mol PCl₅/4 mol H₂O = 0.102 mol PCl₅
Part 1(Picture 1):
Peptides isolated from rapeseed that may lower blood pressure have the following sequence of amino acids.
Part A
At physiological pH the N-terminus of an amino acid exists as the ammonium ion, and the C-terminus exists as the carboxylation ion.
Draw the structure of Arg-Ile-Tyr.
Draw the molecule on the canvas by choosing buttons from the Tools (for bonds and charges), Atoms, and Templates toolbars, including charges where needed.
Part 2(Picture 2):
Part A
What are the amino acids in the peptide?
Spell out the full names of the compounds. Enter your answers separated by a comma.
Part B
How would you name the dipeptide in the peptide?
Spell out the full name of the compound.


Answers
The structure of Arg-Ile-Tyr is given below:
What are Peptides?Peptides make up short chains of amino acids, which act as the fundamental units for building proteins. Within these chains, peptides usually consist of no more than 50 amino acids in contrast to proteins that are made up of significantly longer structures.
Found throughout various sources such as plants, animals, and bacteria, peptides play crucial roles in multiple biological processes including signaling, enzyme activity, and immune response.
Additionally, artificially synthesized peptides serve several purposes within the medicine, cosmetics, and food industries. Scientists are also exploring certain peptide's potential therapeutic advantages mainly pertaining to anti-inflammatory, antimicrobial, and anticancer activities.
Read more about peptides here:
https://brainly.com/question/21884818
#SPJ1


A 500.0 g sample of aluminium, I initially at 25.0 degrees, absorbs heat from its surroundings and reaches a final temperature of 90.7 degrees. How much heat (in KJ) has been absorbed by the sample? To one decimal place
Specific heat= 0.9930j g-1 K-1 for aluminium
Answers
Q= 32620.05
A 500.0 g sample of aluminum, initially at 25.0 degrees, absorbs heat from its surroundings and reaches a final temperature of 90.7 degrees. 32.62245 kJ heat has been absorbed by the sample.
What is specific heat?The term specific heat is defined as the amount of heat required to increase the temperature of 1 gram of a substance 1 degree Celsius (°C).
To calculate the amount of heat absorbed by the sample, use the formula:
Q = mcΔT
where Q is the amount of heat absorbed by the sample, m is the mass of the sample, c is the specific heat of aluminum, and ΔT is the change in temperature of the sample.
Substituting the given values into the formula, we get:
Q = 500.0 g × 0.9930 J/g·K × (90.7°C - 25.0°C)
Q = 500.0 g × 0.9930 J/g·K × 65.7 K
Q = 32,622.45 J
To convert the result to kilojoules (kJ), we divide by 1000:
Q = 32.62245 kJ
Thus, the amount of heat absorbed by the sample is 32.6 kJ.
To learn more about the specific heat, follow the link:
https://brainly.com/question/11297584
#SPJ3
Answer this correctly please...
1. Two body systems Which of the following is NOT a part of the integumentary system of the body?
A. hair
B. bones
C. skin
D. finger and toe nailswork together to help remove waste products from blood.
2. What are these two systems?
A. circulatory and excretory
B. skeletal and digestive
C. circulatory and integumentary
D. muscular and excretory
3. One of the functions of the endocrine system in the body is to -
A. circulate blood to all parts of the body
B. collect waste products and remove them from the body
C. provide a strong framework for the body
D. produce enzymes to help digest food
6. When a person’s body needs food, the brain helps maintain homeostasis by sending signals that make the person
A.feel hungry.
B. perspire.
C. put on a sweater.
D. feel tired
7. The largest human body organ which regulates temperature and serves as a barrier against harmful microorganisms belongs to the -
A. circulatory system
B. nervous system
C. digestive system
D. integumentary system
8. Which of the following is NOT a part of the respiratory system?
A. diaphragm
B. esophagus
C. lungs
D. trachea
9. What would happen to your body if you had little or no bone marrow?
A. Your other systems would make up for it
B. You would not have enough red blood cells
C. Nothing would happen to you.
D. You would not have enough cartilage.
Answers
Answer:
1. B
2. C
3. B or D
6. A
7. D
8. A
9. A
Explanation:
Brainliest plz answered to the best of my abilitys
Use the data below to calculate ΔH°rxn for the reaction MgO(s) + CO2(g) à MgCO3(s).
ΔH°f: MgO(s) = -602 kJ mol-1
, CO2(g) = -394 kJ mol-1 and MgCO3(s) = -1096 kJ mol-
Answers
The ΔH°rxn for the reaction MgO(s) + CO2(g) à MgCO3(s) is -100. kJ mol-1.
What is enthalpy ?
Enthalpy is a property or state function that resembles energy; it has the same dimensions as energy and is therefore measured in joules or ergs. The value of enthalpy is solely dependent on the temperature, pressure, and composition of the system, not on its history.
Using ΔH° = ∑ΔfH°(products) – ∑ΔfH°(reactants), the enthalpy change is:
ΔH° = ΔfH°(MgCO3(s)) – [ΔfH°(MgO(s)) + ΔfH°(CO2(g))]
= (-1096 – [-394 -602]) kJ mol-1
= -100. kJ mol-1
To learn more about enthalpy click on the link below:
https://brainly.com/question/27207707
#SPJ9
Observation made when a sealed vessel containing dry ice is opened at room temperature
Answers
Answer:
Dry ice is the name for carbon dioxide in its solid state. At room temperature, it will go from a solid to a gas directly. While carbon dioxide gas is invisible, the very cold gas causes water vapor in the air to condense into water droplets, thus creating fog.
At which part of a river would you have the best luck growing food?
A. Bend
B. Delta
C. Alluvial fan
D. Source
Answers
Answer:
B. Delta
Explanation:
b. Use the Vander Waal equation to calculate the pressure of 1.00 mol of Cl₂ in a volume of 30.0L at a temperature of 600.0K. And determine the relationship between the Vander Waal and the ideal gas equation. (Please check the Vander Waal constants online) [5 marks]
Answers
The value of the pressure calculated from the Vander Waal equation is slightly less than that is calculated from the ideal gas equation.
What is the pressure?We know that the ideal gas equation assumes that there is no interaction that exists between gas molecules. The ideal gas equation corrects the ideology with the addition of constants that take care of possible interaction between gases.
Now, using the ideal gas equation;
PV =nRT
P = ?
V = 30.0L
T = 600.0K
n = 1.00 mol
R = 0.082 atmLK-1mol-1
P = nRT/V
P = 1.00 mol * 0.082 atmLK-1mol-1 * 600.0K/30.0L
P = 1.64 atm
Using the Vander Waal equation
a = 6.49
b = 0.0562
Thus;
P = RT/V - b - a/V^2
P = 0.082 * 600.0/30.0 - 0.0562 - 6.49/(30)^2
P = 49.2/29.9438 - 6.49/900
P = 1.64 - 0.00721
P = 1.63 atm
Thus the value of the pressure calculated from the Vander Waal equation is slightly less than that is calculated from the ideal gas equation.
Learn more about ideal gas equation:https://brainly.com/question/4147359
#SPJ1
A sample of 8.5 g NH3 on oxidation produces 4.5 g of NO. Calculate the percent yield.
Reaction: 4 NH3(g) + 5 O2(g) → 4 NO(g) + 6 H2O(g)
Answers
Answer:
The answer is 30%
For the following reaction:
2 N2O5 → 4 NO2 + O2
The rate of formation of oxygen is 0.200 mol/L. The rate of formation of NO2 will be
Group of answer choices
0.0500 mol/L
0.200 mol/L
0.500 mol/L
0.800 mol/L
Answers
The rate of the formation of nitrogen dioxide can be obtained as 0.800 mol/L. Option D
What is rate of reaction?The rate of reaction depends on various factors, including the nature of the reactants, the concentrations of the reactants, the temperature, the presence of catalysts, and the surface area of the reactants. It is determined by the collision of particles and their energy, orientation, and the effectiveness of the collision in breaking and forming chemical bonds.
We have that;
1/4d[\(O_{2}\)]/dt = d[\(NO_{2}\)]/dt
Thus;
Rate of formation of the nitrogen dioxide = 2 * 0.200 mol/L
= 0.800 mol/L
Learn more about rate of reaction:https://brainly.com/question/30546888
#SPJ1
Which sense organ detects vibrations in the air. A. Ear B. Eye C. Tongue D. Nose
Answers
What mass
(in kg) does 5.84 moles of titanium (Ti) have?
Answers
Answer:
so for every 1 kg it's 10 to the three. So we multiply 5.84 times 47.867 divided by 10 to the three, which is just 1000. Doing that should give you 0.280 kg of titanium.
Explanation:
The mass of 5.84 moles of titanium (Ti) is 0.279 kg.
What are moles?Moles can be defined as the amount of substances present in a system that contain the same number of entities as the number of atoms in 12g of carbon 12.
Given moles of Titanium = 5.84 moles
Molar mass of Titanium = 47.867 g
Mass = Moles x Molar mass
Mass of Titanium = 5.84 x 47.867
= 279.54g
To convert in kgs = 279.54 / 1000
= 0.279kg
Hence, the mass of 5.84 moles of titanium (Ti) is 0.279 kg.
To learn more about moles here
https://brainly.com/question/15209553
#SPJ2
Explain the production of salt
Answers
Answer: Sodium chloride forms from the ionic bonding of sodium ions and chloride ions. There is one sodium cation (Na+) for every chloride anion (Cl–), so the chemical formula is NaCl
Explanation:
